Weg met de resistentie door licht en zuurstof

Aangezien bacteriën steeds vaker resistent worden tegen antibiotica is het belangrijk om die geneesmiddelen zo weinig mogelijk te gebruiken. Wiktor Szymanski, hoogleraar Medische Chemie, Fotofarmacologie en Beeldvorming, werkt daarom samen met collega’s uit het UMCG en de Faculty of Science and Engineering om bacteriële infecties met behulp van licht op te sporen en op te ruimen. ‘Daar zal ik de komende twintig jaar nog wel mee bezig zijn.’
FSE Science Newsroom | Tekst René Fransen | Beeld Leoni von Ristok
Dit is het tweede artikel in een serie van drie over antibiotica resistentie. Het eerste artikel beschrijft nieuwe manieren om infecties te bestrijden. In het derde artikel staat hoe ecologie en evolutie kunnen helpen om resistentie te doorbreken.
Mijn doel is de bacterie snel en op de juiste plek aan te pakken
Wanneer een orthopedische patiënt bijvoorbeeld een kunstknie krijgt kan dat leiden tot complicaties. ‘Als de operatiewond niet geneest kan dat komen door een reactie op het materiaal van de kunstknie, of het is een bacteriële infectie’, vertelt Szymanski. Het duurt vervolgens een paar dagen om uit te zoeken welke bacterie de infectie veroorzaakt. Daarom is het gebruikelijk om de patiënt alvast een breed-werkend antibioticum te geven, maar dit kan het ontstaan van resistentie in de hand werken. Szymanski wil daarom de diagnose versnellen met behulp van beeldvormende technieken, en daarnaast de bacteriën aanvallen met behulp van licht. ‘Mijn doel is de bacterie snel en op de juiste plek aan te pakken.’
Bacteriën zijn ruwweg in te delen in twee groepen die elk een andere behandeling vragen: Gram-positief en Gram-negatief. Om snel te achterhalen welk van deze twee groepen de infectie veroorzaakt gebruikt Szymanski het antibioticum vancomycine, dat alleen bindt aan Gram-positieve bacteriën. Samen met de onderzoeksgroep van UMCG hoogleraar Jan Maarten van Dijk heeft hij een fluorescerend molecuul aan dit antibioticum gekoppeld, waardoor het rood oplicht wanneer je er met een groen licht op schijnt. Op die manier kan hij Gram-positieve bacteriën opsporen.
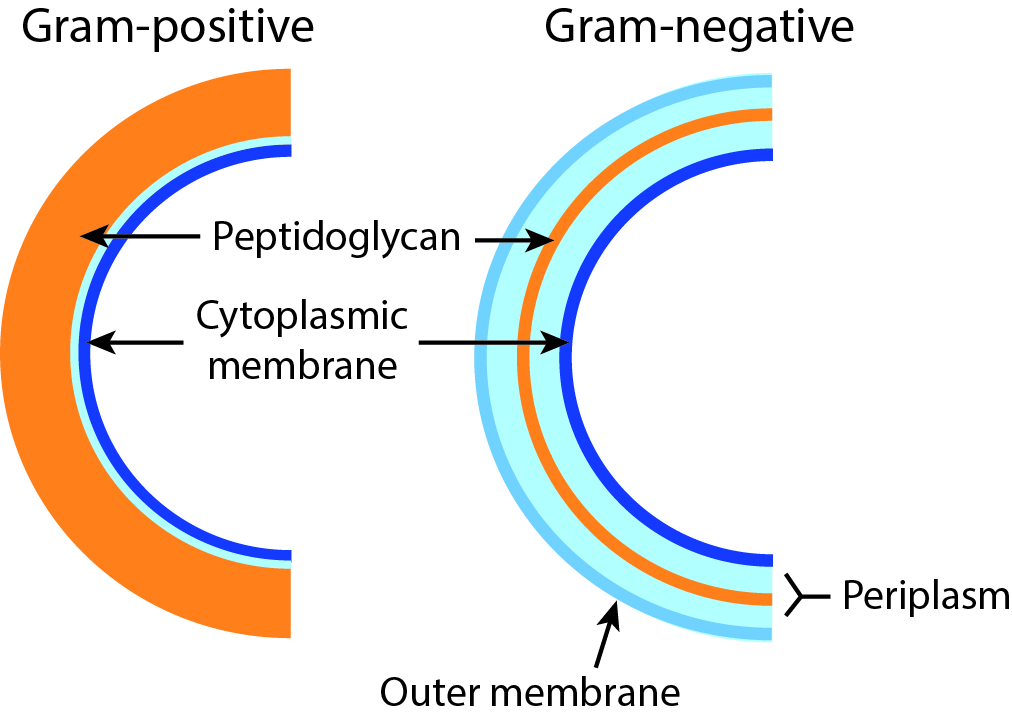
Gram-negatief en Gram-positief
Bacteriën zijn grofweg te verdelen in twee klassen: Gram-negatief en Gram-positief, gebaseerd op een kleurstof die sommige bacteriën paars kleurt, en andere rood. De reden daarvoor is het verschil in de samenstelling van de celwand. Gram-positieve cellen hebben een dikke laag van een molecuul genaamd peptidoglycaan aan de buitenkant van de celmembraan. Gram-negatieve cellen hebben een tweede membraan die de peptidoglycaan-laag bedekt, waardoor de kleuring minder sterk is, wat een lichtere (rode) kleur oplevert. Veel antibiotica hebben juist die laag peptidoglycaan als doelwit, waardoor ze minder effectief zijn tegen Gram-negatieve bacteriën, waarbij deze is afgedekt met de tweede membraan.
Eenvoudiger belichten
Om met licht een geïnfecteerde plek in het lichaam te vinden is het nodig om een glasvezelkabel naar binnen te schuiven. Maar Szymanski heeft een alternatieve beeldvormende techniek ontwikkeld waarbij dat niet nodig is. Dat deed hij samen met hoogleraar organische chemie Ben Feringa en UMCG hoogleraar PET en Radiochemie Philip Elsinga. In plaats van licht wordt hierbij de zogeheten positron emissie tomografie ingezet. Hiervoor verbonden de onderzoekers een radioactief fluor-atoom aan het eerder genoemde antibioticum vancomycine. Door radioactief verval zendt dit atoom gammastraling uit, die zichtbaar is in een speciale medische scanner. Szymanski: ‘Het radioactieve antibioticum geven we in een zeer lage dosis, waar de patiënt geen last van heeft maar waarmee we wel de bron van de infectie in beeld kunnen brengen.’
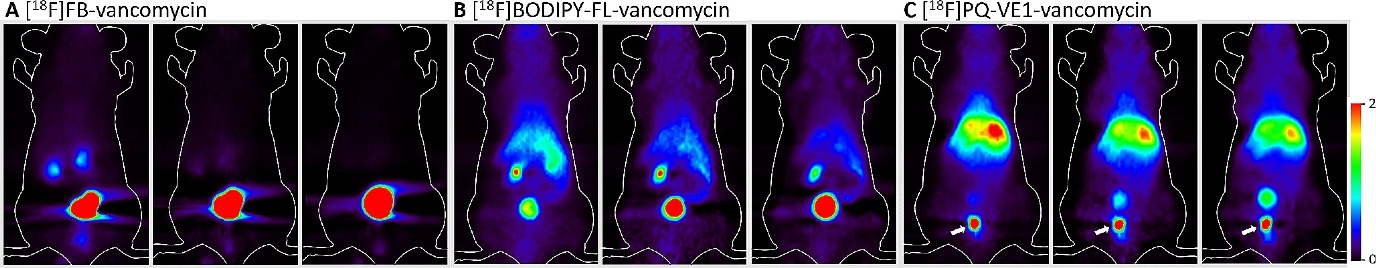
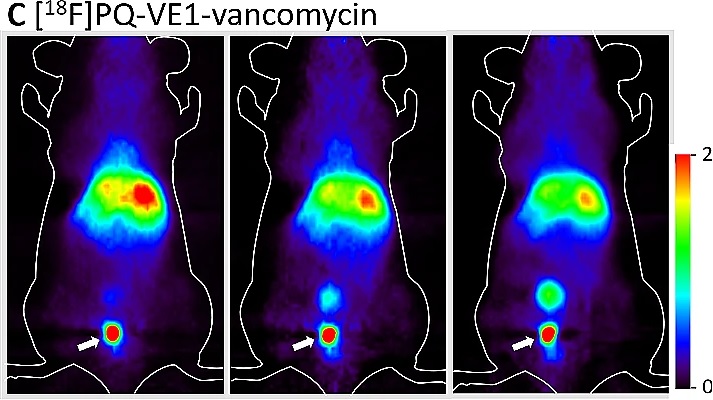
Aanvallen met licht
Voor zover wij nu weten, lijkt het onwaarschijnlijk dat bacteriën resistent worden tegen deze behandeling
Het lokaliseren en identificeren van de infectie is natuurlijk de eerste stap, die moet worden gevolgd door een behandeling op precies de juiste plek. Daarvoor hebben de onderzoeksgroepen van Szymanski en Van Dijk hun fluorescerende vancomycine aangepast om een zeer reactieve vorm van zuurstof af te geven, die alles dat op zijn weg komt verbrandt. ‘Dit zogeheten singletzuurstof kan bacteriën doden. En voor zover wij nu weten, lijkt het onwaarschijnlijk dat bacteriën resistent worden tegen deze behandeling.’
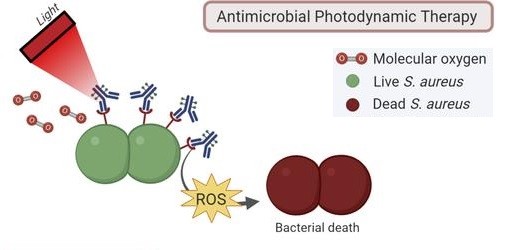
Een andere benadering is om de patiënt een gewoon antibioticum te geven, maar dan uitgerust met een lichtgevoelige schakelaar waarmee artsen het geneesmiddel ‘aan’ kunnen zetten op de plek van de infectie. ‘En omdat het zichzelf na een paar uur weer uitschakelt is de blootstelling van de patiënt aan het antibioticum veel lager dan gebruikelijk.’ Dit verkleint de kans dat bacteriën op andere plekken in het lichaam resistent worden, en het voorkomt dat de patiënt een actief antibioticum uitscheidt in het riool.
Al dit werk vindt nog plaats in onderzoekslaboratoria. Het zal nog flink wat tijd kosten om deze methoden klaar te maken voor gebruik in het ziekenhuis. Syzmanski: ‘We zullen eerst moeten aantonen dat deze methoden ook echt gaan werken. Hoe kunnen we bijvoorbeeld licht schijnen op plekken diep in het lichaam? En als het werkt moeten we bewijzen dat het veilig is voor de patiënten.’ Zijn ultieme doel is om beeldvorming en het opruimen van de infectie in één behandeling te combineren. ‘Daar zal ik vermoedelijk de komende twintig jaar van mijn carrière nog wel mee bezig zijn.’
Dit is het tweede artikel in een serie van drie over antibiotica resistentie. De anderen lees je hier:
Alleen naar ziekteverwekkers en genen kijken is te beperkt in de strijd tegen antibioticaresistentie, stelt microbioloog Marjon de Vos. Bacteriën die op het eerste gezicht onschuldige omstanders lijken, blijken een belangrijke rol te spelen in de ontwikkeling van resistentie.
Er zijn dringend nieuwe antibiotica nodig, omdat steeds meer bacteriën resistent zijn voor de huidige geneesmiddelen. RUG-onderzoekers Dirk-Jan Scheffers en Marthe Walvoort werken aan nieuwe manieren om de resistentie te doorbreken, door gaten te maken in bacteriën, of hun celwand te verzwakken.
Meer nieuws
-
17 februari 2026
De lange zoektocht naar nieuwe fysica
-
10 februari 2026
Waarom slechts een klein aantal planeten geschikt is voor leven
