Beating resistance using light and oxygen

Since bacteria are becoming increasingly resistant to antimicrobial agents, it is important to use these agents as sparingly as possible. Wiktor Szymanski, Professor of Medicinal Chemistry, Photopharmacology and Imaging, together with his colleagues at the UMCG and the Faculty of Science and Engineering, uses light to localize and attack bacterial infections. ‘This is what I’ll devote the next 20 years to.’
FSE Science Newsroom | Text René Fransen | Images Leoni von Ristok
This is the second article in a series of three on how to deal with antimicrobial resistance. The first described new ways to fight infections and the third article will dive into the importance of ecology and evolution in preventing resistance.
My aim is to hit the bacteria quickly and in the right location
When a patient undergoes orthopedic surgery, such as getting a new knee implant, it may lead to complications. ‘If the knee doesn’t heal, this could be a reaction to the artificial joint, or it may be a bacterial infection,’ Szymanski says. It takes a few days to find out which bacteria are causing the infection, which is why in current medical practice a broad-spectrum antibiotic is often given initially. However, this contributes to the global problem of resistance in bacteria. Szymanski wants to speed up diagnosis with the help of imaging techniques, but also attack the bacteria using light. ‘My aim is to hit the bacteria quickly and in the right location.’
Bacteria can be roughly divided into two groups: Gram-positive and Gram-negative, each requiring different treatment. To quickly detect which of these two groups is present in the infected area, Szymanski uses an antibiotic called vancomycin that only binds to Gram-positive bacteria. Together with the research group of UMCG Professor Jan Maarten van Dijl, he added a fluorescent substance to this antibiotic, causing it to emit red light when green light is shone on it. This enables the detection of the Gram-positive bacteria.
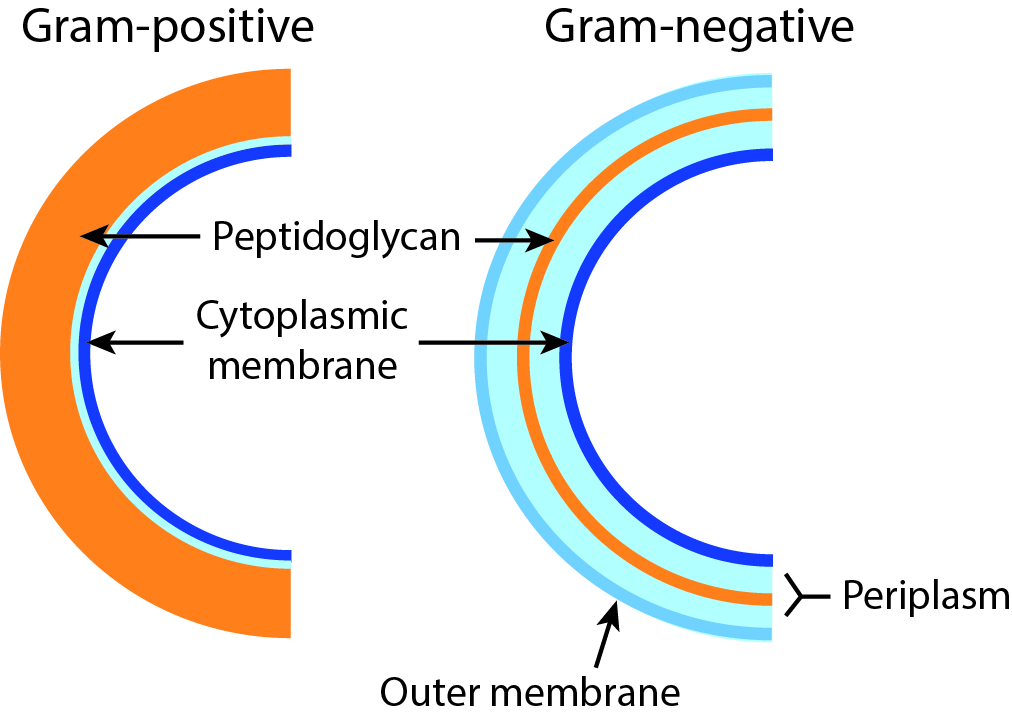
Gram-negative and Gram-positive
Bacteria can be divided into two classes: Gram-negative and Gram-positive, based on a dye that makes some bacteria turn purple and others red. The reason for this difference lies in the composition of the cell envelope. Gram-positive cells have a thick layer made up of a molecule called peptidoglycan on the outside of the cell membrane. Gram-negative cells have a second membrane covering the peptidoglycan layer, which causes the discolouration–caused by the dye–to be less pronounced, resulting in a lighter (red) colour. Many antibiotics target the peptidoglycan layer, but these tend to be less effective against Gram-negative bacteria, because the layer in these cells is protected by the second membrane.
Shining light more easily
To shine light on the deeply infected area, and to detect it, it is necessary to insert a fibre optic cable into the body. To avoid this, Szymanski developed an alternative imaging method, together with Prof. Ben Feringa (FSE) and Prof. Philip Elsinga (UMCG). Instead of light, positron emission tomography (PET) is used. This is done by attaching a radioactive fluoride or carbon atom to the previously mentioned antibiotic vancomycin. Via radioactive decay, this atom will cause the emission of gamma radiation that can be detected in a medical scanner. Szymanski: ‘The radioactive antibiotic is given in a low dose, which does not harm the patient but allows us to see the source of the infection.’
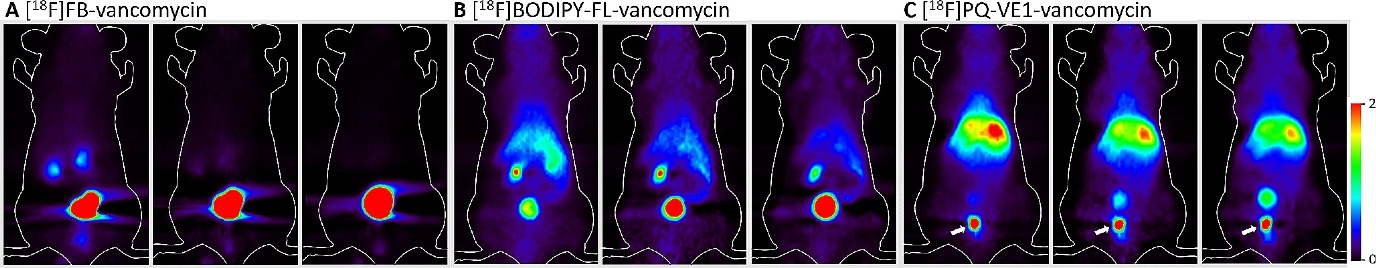
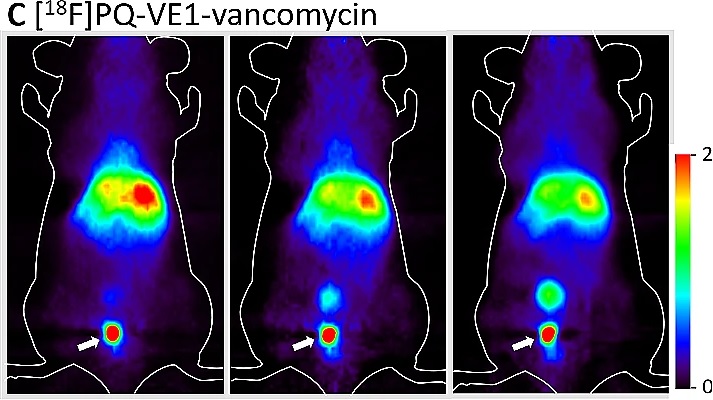
Using light to attack
As far as we know, it seems unlikely that bacteria could become resistant to this type of treatment.
Locating and identifying the infection, however, is only the first step and should be followed by localized treatment. To do this, the Szymanski and Van Dijl research groups modified the light-emitting vancomycin to instead produce a highly reactive type of oxygen that ‘burns’ everything it collides with. ‘This so-called singlet oxygen can kill the bacteria, and, as far as we know, it seems unlikely that bacteria could become resistant to this type of treatment.’
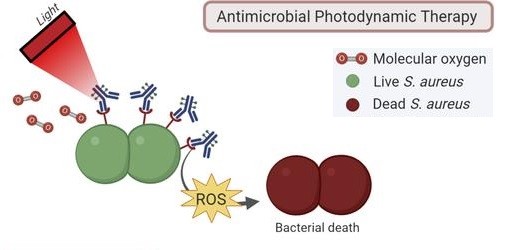
A second approach is to treat the patient with a regular antibiotic equipped with a light-sensitive switch that allows doctors to switch it on at the area of the infection. ‘And it also switches itself off again after a few hours, so the patient’s exposure to this antibiotic is greatly reduced.’ This diminishes the chance that bacteria elsewhere in the body become resistant, and it prevents an active antibiotic from ending up in wastewater after the patient has excreted it.
All this work is being done in research labs, and it will take a lot of time before these methods are ready for use in the clinic. Szymanski: ‘First, we have to demonstrate that these techniques actually work. For example, how can we shine a light deep inside the body? And once it works, we need to prove that it’s safe for patients.’ His ultimate goal is to combine diagnostic imaging and eradication of the infection in one treatment. ‘That’s what I will devote the next twenty years of my career to.’
This is the second article in a series of three on how to deal with antimicrobial resistance. Read the others here:
Looking only at disease-causing bacteria and genes is too limited in the battle against antimicrobial resistance, says microbiologist Marjon de Vos. It turns out that bacteria that were seen as innocent bystanders play an important role in the development of resistance.
We need new antibiotics, as more and more bacterial species are becoming resistant to the existing drugs. University of Groningen scientists Dirk-Jan Scheffers and Marthe Walvoort work on novel ways to beat the resistance by punching holes in bacteria or otherwise weakening their cell wall.
More news
-
29 January 2026
Microplastic research - media hype or real danger?
-
27 January 2026
ERC Proof of Concept grant for Maria Loi
