How to make batteries safer

There is a small chance that rechargeable lithium batteries can ignite, explode, or leak corrosive electrolyte. This can happen spontaneously, most often while charging. However, misuse, poor-quality cells, or physical damage can significantly increase the risk. Giuseppe Portale, associate professor of Polymer Chemistry and Physics at the University of Groningen, is working on safer batteries.
FSE Science Newsroom | René Fransen
Commercial lithium-ion batteries contain a liquid, known as the electrolyte, which allows charged lithium ions to move quickly between the positive and negative electrodes. The electrolyte thus facilitates the chemical reactions that generate electricity and allow the battery to charge and discharge. ‘However, in lithium-ion batteries, this liquid is toxic, corrosive, and flammable,’ explains Portale.
New properties
The problems with liquid electrolytes can be solved by using a solid alternative. However, in a conventional solid, charged lithium ions cannot move as freely as they do in a fluid, resulting in poor battery performance. Portale is therefore working on a more subtle improvement: he creates soft, solid-like electrolytes from long, spaghetti-like molecules called polymers. When mixed with lithium salts, the polymers form a paste-like substance that allows for greater movement of lithium ions than classical, ordered solid electrolytes, though still less than in a liquid.
Our scientists can monitor online how the new batteries perform and how long they last
One major advantage of polymers is that they can be easily modified to give them new properties. Portale created a “lithium highway” along the polymers, where the “asphalt” is composed of oxygen atoms placed along the spaghetti-like strands. His research group tests these innovations in coin cell batteries, which are charged and discharged continuously until they break down. The tests run for weeks or months on a battery tester loaded with dozens of coin cells. ‘Our scientists can monitor online how the new batteries perform and how long they last,’ says Portale.


A problem with polymers
Battery manufacturers are not yet willing to work with polymers, which means that the adoption of solid polymer electrolytes remains limited. Therefore, Portale is also working on an innovation that is easier to adopt: ‘We are experimenting with using polymer electrolytes as coatings on the electrodes, as a way to prevent some of the problems associated with the liquid electrolyte’, says Portale.
One major problem with current lithium batteries is the formation of lithium crystals, especially during rapid charging. These crystals, known as dendrites, can grow so long that they connect the poles and thus short-circuit the battery. A polymer coating that suppresses dendrite formation can prevent this.
The advantages of ‘spaghetti batteries’
The polymer electrolytes are slightly more expensive than the commonly used liquid electrolytes. But once you decide to replace the liquid with polymers, significant improvement can be achieved. By linking individual polymer strands, a network is created that further improves the transport of lithium ions: they can easily hop from one strand to the other to travel between the poles of the battery, provided that the spaghetti-like polymers are flexible enough. Other improvements can even make the polymer green and sustainable.
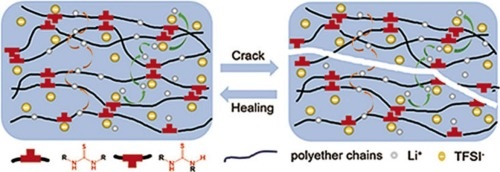
‘We can also add certain particles to the polymers that make them nearly non-flammable,’ says Portale. So, no more exploding smartphones or e-bikes. Other additives help the polymer electrolyte absorb shocks, preventing damage to your phone if it is dropped, and thus reducing the risk of battery explosion. ‘We are even developing self-healing electrolytes, so that any damage can repair itself rather than become permanent.’
You can find the other installments in this series in the box below.
Researchers Xin Sun and Xiaohua Li look at the life cycle of batteries, from raw materials to disposal at the end of battery life, and how to make recycling more effective and greener.
Edwin Otten is working on a battery that stores electric power in large containers of fluid. To make such a battery effective and affordable, a lot more research is still needed.
A great deal of research is required to develop the best or cleanest batteries. But how does a battery actually work?
An €800 million programme funded by the Dutch National Growth Fund aims to stimulate the Dutch battery ecosystem and make a global impact. Scientists from the University of Groningen contribute to this programme, designing and building the batteries of the future.
More news
-
17 February 2026
The long search for new physics
-
10 February 2026
Why only a small number of planets are suitable for life
