How do batteries work, and can they be improved?

Batteries can convert electricity into chemical power, which can then be released to supply electric power. These batteries are in your phone, a torch, or in your car, as an accumulator is a battery too. The extensive use of batteries for the storage of electric power from wind or solar farms, calls for new types that are more efficient, cheaper and more environmentally friendly. Scientists at the UG work on these new batteries, but also on recycling the ones that have been discarded. In a series of five articles, you will learn about the battery developers of the Faculty of Science & Engineering (FSE).
FSE Science Newsroom | René Fransen
A lot of research is needed to develop the best or cleanest batteries. For example, the method with which the lithium in your phone battery, electric bike, or car is obtained is not very environmentally friendly. That is why researchers at the FSE are working on alternatives, in which lithium is replaced by sodium for example, which is much easier to extract. They are also looking for alternatives to rare materials, mainly to avoid being dependent on the few countries in the world where they are mined.

What do batteries consist of?
Batteries can be made with countless combinations of materials. A battery has at least three important components:
-
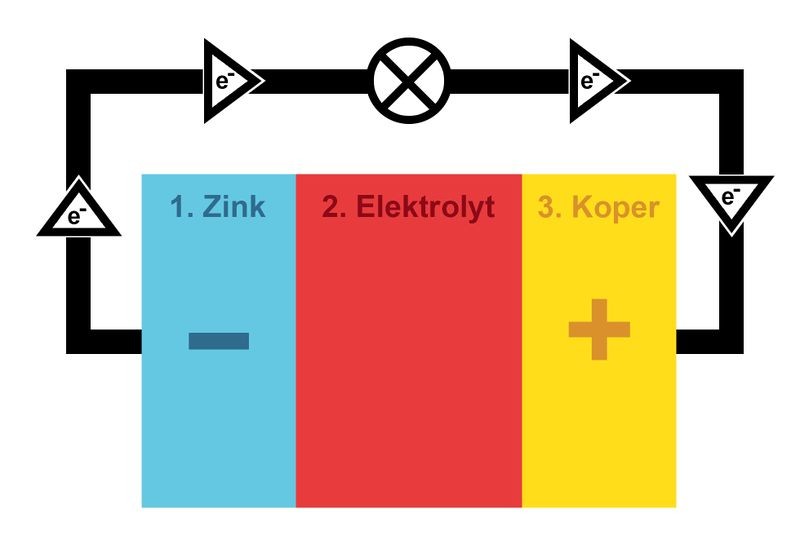
The negative terminal, or anode, where the particles that can emit electrons are located.
-
The positive terminal, or cathode, which contains particles that can absorb the emitted electrons.
-
The electrolyte; a fluid or paste that allows charged particles to pass between the terminals. This is also the substance which enables the chemical reaction in a battery, allowing it to supply electric power or charge.
Anodes are usually composed of graphite, a type of carbon, but can also be made from metals such as aluminium, zinc, or magnesium. Cathodes often contain lithium but also manganese, silver or graphite. With a copper coin and a nail, you can even make a battery of a potato!
There are all kinds of batteries. Some even consist of two large containers of liquid connected to a reactor which charges one container positively and the other negatively. It is not ideal for your car, but it is useful for storing large quantities of surplus electric power from wind farms, for example. At the Faculty of Science and Engineering, Prof Edwin Otten works on such battery.

How does the electric power flow in a battery?
When you switch on a battery-powered device, a path is created that allows electrons to flow from the negative to the positive terminal. If that path passes through the light of your torch, the flow of electrons will cause it to glow.
But how is it possible for the negative terminal to emit electrons? The anode consists of atoms that emit electrons through oxidation, a chemical reaction which occurs between the anode and the electrolyte, the fluid between the terminals. At the same time, the cathode’s side undergoes a reversed reaction called ‘reduction’, enabling it to absorb electrons. This results in a flow of electrons moving from one terminal to the other. The opposite process occurs when charging.
When electrons pile up in the anode, they will repel each other due to their shared negative charge. They then want to move to an area with fewer free electrons. When a battery is placed in an electric circuit, such as a torch, the electrons will enter the circuit through the anode and move towards the cathode, the positive terminal.
Therefore, generating electric power entails a continuous chemical reaction that supplies electrons. This means that, when a battery is discharged — for instance, when using a laptop or phone — there is a constant chemical reaction providing a flow of electrons. However, this reaction ends when the substances required for the chemical reaction are depleted. The battery is now empty.
In a rechargeable battery, two kinds of chemical reactions can take place: one where electric power is produced through oxidation at the anode, releasing electrons, and the other where energy is stored while the battery is being charged. In the latter, the anode absorbs electrons again through a reaction called ‘reduction’. Once the battery is charged, it can supply electric power once more. The reactions involving the release or storage of electric power, are called ‘electrochemical reactions’.
A problem with some batteries is the occurrence of unwanted reactions in the electrolyte. In rechargeable lithium-ion batteries, lithium can be deposited from the electrolyte onto the electrode while charging. This results in the growth of lithium dendrites, which lead to a short-circuit in the battery. It primarily happens with fast charging. At FSE, Guiseppe Portale is working on a solution to prevent this, which would also increase the durability and charging speed of rechargeable batteries.
But there are more unwanted reactions that could occur. That is why, when it comes to scientific research into batteries, it is important to be able to identify all the electrochemical reactions that take place. This research is also done in Groningen by, among others, Prof. Moniek Tromp. Furthermore, FSE scientists experiment with new materials which could yield the desired reaction, and research the environmental impact and recycling of various batteries.
You can find the next installments in this series in the box below.
Researchers Xin Sun and Xiaohua Li look at the life cycle of batteries, from raw materials to disposal at the end of battery life, and how to make recycling more effective and greener.
There is a small chance that rechargeable lithium batteries will ignite or even explode. Giuseppe Portale, associate professor in Polymer Chemistry at the University of Groningen, is working on safer batteries.
Edwin Otten is working on a battery that stores electric power in large containers of fluid. To make such a battery effective and affordable, a lot more research is still needed.
An €800 million programme funded by the Dutch National Growth Fund aims to stimulate the Dutch battery ecosystem and make a global impact. Scientists from the University of Groningen contribute to this programme, designing and building the batteries of the future.
More news
-
29 January 2026
Microplastic research - media hype or real danger?
-
27 January 2026
ERC Proof of Concept grant for Maria Loi
