Breaking the resistance: Why bacteria are stronger together
We know that frequent use of antibiotics can cause resistance in bacteria, and we know how they pass on resistance genes to each other. But simply studying disease-causing bacteria and genes is too limited, says microbiologist Marjon de Vos. Her research takes a broader view of the bacterial ecosystem as a whole. It turns out that bacteria that were considered harmless bystanders play an important role in the development of resistance.
FSE Science Newsroom | Text René Fransen | Images Leoni von Ristok
This is the third article in a three-part series about antimicrobial resistance. The first article describes new ways to fight infections, and the second article describes new ways to use antibiotics with switches and fluorescent labels for targeted treatment.
De Vos is convinced that our approach to infections is overly reductionist. ‘When I was working on my postdoc, the general consensus was that the bacterium E. coli was the main cause of urinary tract infections’, she recalls. The existence of a bacterial community — the microbiome — in the urinary tract was still unknown at the time. All of that has changed now, partly thanks to De Vos’s work.
A stable bacterial ecosystem
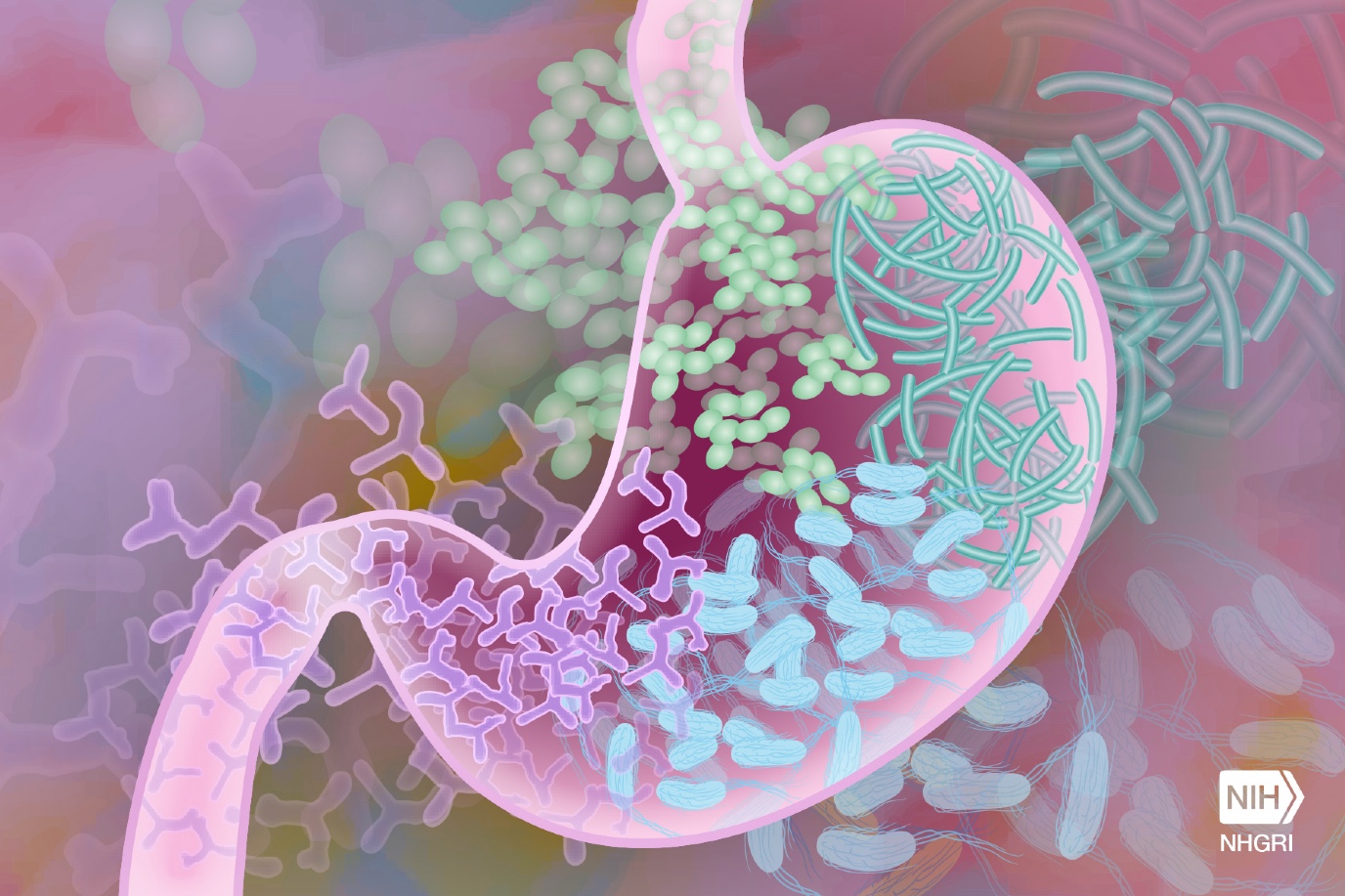

With the help of a British and German microbiologist, De Vos discovered a stable bacterial ecosystem in urine samples of infected women. Initially, it was thought that these samples were simply contaminated with bacteria other than E. coli, the usual suspect. However, it turned out that the other bacteria were not contaminants but rather a collection of bacteria that formed a stable ecosystem in the urinary tract. De Vos: ‘The antibiotics administered to combat E. coli disrupted this ecosystem. But after a while, it returned to its original composition, causing the urinary tract infection to recur.’
After all, a bacteria is not always a pathogen
Simply focusing on the pathogen, E. coli in this case, is not a good approach, De Vos argues. ‘After all, a bacteria is not always a pathogen. For example, E. coli bacteria normally live in the gut, where they are harmless’, says De Vos. Moreover, the composition of the bacterial ecosystem also plays a role. In the ‘contaminated’ samples, the presence of certain Gram-positive bacteria meant that E. coli could develop resistance much more quickly, and the resistance genes also spread more rapidly.
Selecting antibiotics based on the ecosystem
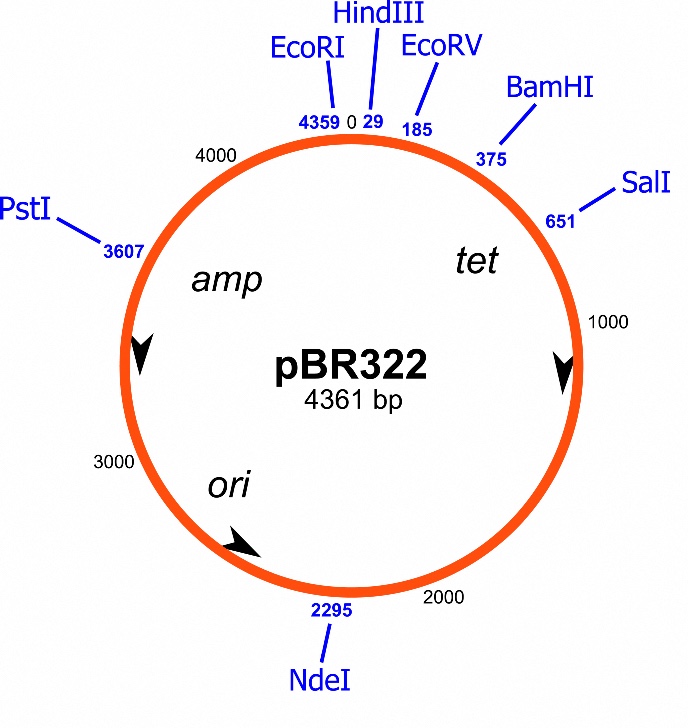
Bacteria are capable of exchanging genetic information, and thus spreading resistance amongst themselves. They do this by exchanging ring-shaped pieces of DNA called plasmids, which usually contain a number of useful genes. These ring-shaped pieces can even be exchanged between bacteria of different species. Microbiologists have identified many such DNA exchange rings containing resistance genes, but again, it is not that simple, according to De Vos: ‘Cells that receive such a resistance plasmid don’t always become resistant. The normal bacterial genes can alter the effect of the resistance genes on the plasmid. And the other bacteria in the urinary tract microbiome can determine how resistant such bacteria are.’
It is important for doctors to be aware of the entire ecosystem, so that they can prescribe the right antibiotic
It is now clear that the bacterial community in the urinary tract helps determine how effective antibiotics are against an E. coli infection. ‘The goal of my research is to understand these kinds of processes,’ says De Vos, who is convinced that this will change the way we look at infections and their treatment. ‘For example, the presence of two specific types of bacteria may reduce the sensitivity of the infection to the standard antibiotic. In that case, it is important for doctors to be aware of the entire ecosystem, so that they can prescribe the right antibiotic. On the other hand, some bacteria in the ecosystem may actually enhance the effect of a specific antibiotic.’
Polluted soil

Bacterial resistance is not only caused by antibiotics. Pesticides and heavy metals from polluted soil can also play a role. ‘For instance, bacteria use special mechanisms to get rid of these heavy metals and other substances, known as efflux pumps, in their cell walls.’ However, these efflux pumps can also excrete antibiotics. ‘So effectively, because of pesticides and contaminated soil, we’ve selected for bacteria with excellent mechanisms for getting rid of antibiotics.’
One Health - a broader view on infections
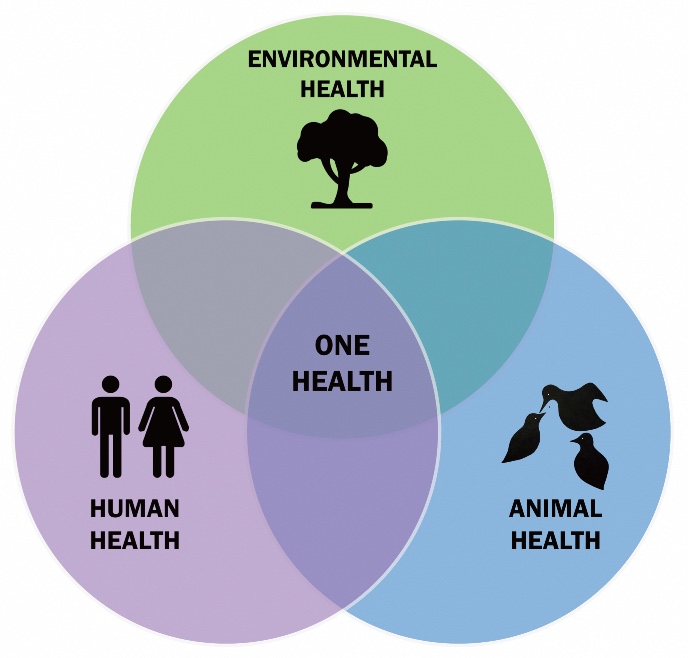
Human diseases are often intertwined with animal diseases, but they are also linked to the wider environment. One example is the influenza virus, which is often transmitted from pigs in intensive livestock farming to humans. Other viruses, such as HIV and SARS-CoV2, have also jumped from wild animals to humans. The similarities between veterinary medicine and human medicine were noted around sixty years ago. And about twenty years ago, the One Health approach was developed, which brought together the health of humans, animals, and the environment.
The One Health approach is used, for example, to analyse how resistance can spread in hospitals, or through agriculture. This approach is also used to systematically monitor, detect, and respond to health threats that arise at the intersection of human, animal, and environmental health, for example to detect potential pandemic threats. However, according to De Vos, even the One Health approach focuses too narrowly on pathogens and resistance genes. ‘This is still a reductionist view. At the Groningen Institute for Evolutionary Life Sciences, we have a broader view, which also takes into account the processes of ecology and evolution.’
Only then can we devise a treatment that restores the balance in these ecosystems
Untreatable infections
De Vos gives a final example of the importance of looking at the bigger picture. When a urinary tract infection is treated with antibiotics, the E. coli bacteria are killed. ‘But then other types of bacteria take their place – sometimes even types that stimulate the regrowth of E. coli!’ The entire bacterial ecosystem must therefore be taken into account in both diagnosis and treatment. ‘Only then can we devise a treatment that restores the balance in these ecosystems.’
De Vos is firmly convinced that we need to understand bacterial ecosystems before we can work on a solution. ‘This is important, because we are still using too many antibiotics, which are causing greater resistance. This could lead to an increase in untreatable infections and therefore to more deaths.’
This is the third article in a three-part series about antimicrobial resistance. Read the other articles here:
As bacteria become increasingly resistant to antimicrobial agents, it is essential to use these agents as sparingly as possible. Wiktor Szymanski, Professor of Medicinal Chemistry, Photopharmacology and Imaging, and his colleagues use light to localize and attack bacterial infections.
We need new antibiotics, as more and more bacterial species are becoming resistant to the existing drugs. University of Groningen scientists Dirk-Jan Scheffers and Marthe Walvoort work on novel ways to beat the resistance by punching holes in bacteria or otherwise weakening their cell wall.
More news
-
17 February 2026
The long search for new physics
-
10 February 2026
Why only a small number of planets are suitable for life
