The ocean absorbs carbon from the air, but what if the temperature increases?

‘Fortunately, seawater absorbs carbon dioxide (CO₂). If it didn’t, things would have been over and done with already,’ according to climate and ocean researchers Richard Bintanja and Rob Middag. ‘The ocean is a massive carbon reservoir.’ But what actually happens to carbon absorption as the climate changes? For example, when glaciers and sea ice melt due to higher temperatures?
FSE Science Newsroom | Text Charlotte Vlek | Images Leoni von Ristok
This article is the second in a series about the carbon cycle: the cycle in which carbon is exchanged between the atmosphere and the ocean, and between plants and rocks. But this carbon cycle is being disrupted by the CO2 added to the atmosphere through human activity. In this article, you can read about the carbon exchange between the atmosphere and seawater.
The amount of carbon dioxide absorbed by seawater depends on how much CO₂ concentrations in the air and in the water differ. Compare it to a warm room and a cold one: heat flows faster into the cold room when the temperature difference is greater. This is how the Earth regulates its own balance: more CO₂ in the atmosphere leads to greater carbon absorption by seawater.
When we think about the oxygen we breathe, we instantly think of the rainforest. But in fact, a large share comes from phytoplankton
However, there is a limit to the ocean’s carbon intake: once the top layer becomes saturated with CO₂, it no longer absorbs more from the atmosphere. The ocean can absorb more carbon from the air only after the existing carbon in surface waters has been transported to the depths. Carbon is transported to deeper waters by currents or by phytoplankton: single-celled plants that take in CO₂ and release oxygen, much like plants on land.
‘When we think about the oxygen we breathe, we instantly think of the rainforest. But in fact, a large share comes from phytoplankton,’ laughs Richard Bintanja, Professor of Climate and Environmental Change at the University of Groningen and the Royal Netherlands Meteorological Institute (KNMI). As dead phytoplankton sink to the bottom, they bring the carbon they absorbed with them. This helps remove carbon from surface waters efficiently.
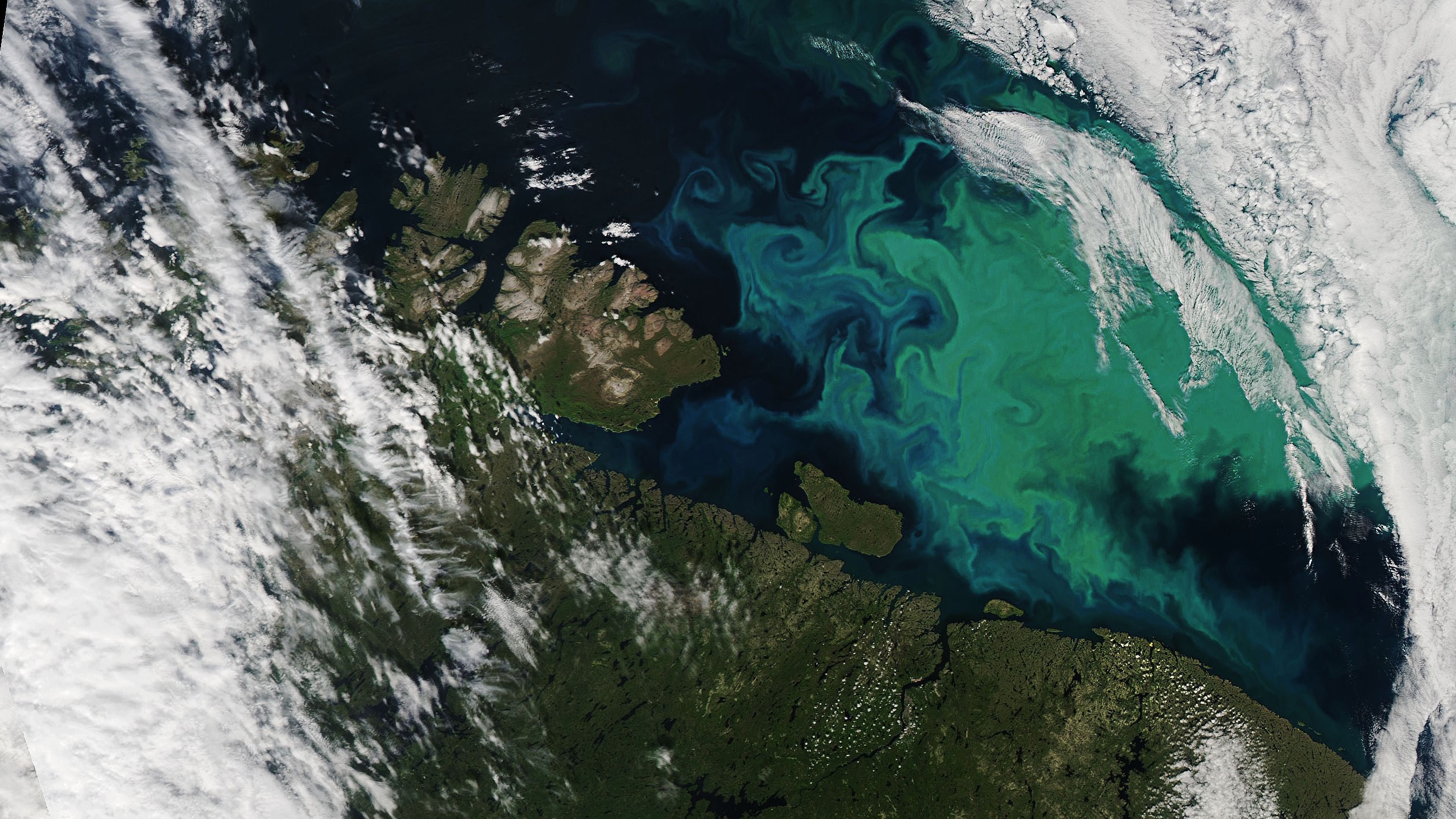
Iron deficiency around Antarctica
‘But to convert CO₂, phytoplankton need iron as a nutrient,’ says Rob Middag, Professor by special appointment at the University of Groningen and the Royal Netherlands Institute for Sea Research (NIOZ). You can notice it instantly when looking at iron-poor seawater in a bottle. Add iron to the water in its correct chemical form, you can’t just add paper clips. Then you can see the water turn green from the phytoplankton.’

Melting glaciers might carry iron into the Southern Ocean, which would in turn support more phytoplankton growth
But iron dissolves poorly in seawater. This means that it precipitates quickly and then easily sinks to the bottom of the ocean. Along the coast, this is less of a problem because enough iron-rich dust enters the sea from the land, and rivers also carry iron. However, in the remote Southern Ocean around Antarctica, iron is scarce. ‘Researchers once thought it would be a good idea to add iron to the ocean there, encouraging more phytoplankton to grow and absorb more carbon. But that proved not viable,’ says Middag (see text box).
Middag himself travelled to Antarctica with his research team on icebreakers, but for a different purpose: ‘We thought that melting glaciers might carry iron into the Southern Ocean, which would in turn support more phytoplankton growth.’ Several PhD students explored this hypothesis and arrived at both positive and less positive conclusions.
Middag: ‘One PhD student studied in the lab how phytoplankton is coping with these changing conditions: more iron, but also higher temperatures due to the melt water. How does that affect growth?’ The good news is that phytoplankton thrives in higher temperatures, provided there is enough iron. The bad news, discovered during the icebreaker expedition, is that the iron from the glacier is in the wrong chemical form and not easily absorbed. So phytoplankton hardly benefits from it. Middag: ‘It’s a bit like someone with an iron deficiency chewing on paper clips: it’s pointless.’
The expedition that brought iron to the sea
‘Groningen scientists were once among the first to participate in a project aimed at adding iron to the ocean to see if it could artificially boost phytoplankton growth,’ says Rob Middag. ‘They joined a German ship to the Southern Ocean.’
In the lab, adding iron led to an increase in phytoplankton. But would it also work in nature? Would there be enough light for photosynthesis, and would the phytoplankton not be eaten too quickly? ‘The expedition was necessary to figure that out,’ says Middag. ‘And they succeeded! At least in the sense that it resulted in so much phytoplankton growth that it was even visible in satellite images from space.’
But doing this only occasionally is pointless, Middag explains, precisely because the iron sinks to the ocean floor. ‘You’d need to add iron continuously to notice any real effect. And that’s rather complicated: you can’t just throw paper clips overboard. The iron must first be mined and processed, then transported by ship to Antarctica, and all of that produces carbon emissions... In the end, it wasn’t viable.’
Melting Arctic sea ice

What you picture in your mind is often oversimplified. Our climate models consistently show that everything is connected, and if you change one thing, everything changes
Bintanja now focuses on the Arctic, though his polar expeditions are largely a thing of the past. Bintanja: ‘You would think that more open water would mean more phytoplankton, and with it, more CO2 absorption.’ That’s because phytoplankton needs sunlight for photosynthesis and therefore cannot grow under sea ice.
But does it actually work like that? ‘What you picture in your mind is often oversimplified,’ Bintanja says. That is why he is using KNMI’s climate models to investigate exactly how this scenario of melting sea ice and phytoplankton could play out. ‘Our climate models consistently show that everything is connected, and if you change one thing, everything changes.’
The current conclusion is that there is no clear conclusion. Different models produce different results. Bintanja explains: ‘Phytoplankton also needs nutrients, such as nitrogen and iron. The plankton itself stays near the surface, but nutrients need to be supplied from the depths by ocean currents. It’s very difficult to model the precise movement of these vertical currents.’
And what about seaweed?
Off the coast of Scheveningen, a seaweed farm sponsored by Amazon aims to reduce the company’s carbon footprint. Seaweed absorbs CO2 from the air as well, and when it sinks into the deep ocean, that carbon is effectively captured from the air.
‘Immoral,’ says Klaas Timmermans, Professor by special appointment at the University of Groningen and NIOZ. ‘When so many people are suffering from hunger, it would be better to grow seaweed for food, rather than sink it to the depths.’ Timmermans is conducting various research projects related to the consumption of seaweed. Among other things, he studies the cultivation of different types of seaweed and how the useful proteins that the plant contains can be processed.
Rob Middag, his colleague at NIOZ, is also highly sceptical. ‘Capturing CO2 with seaweed is such a typically human idea! There’s already a whole ecosystem absorbing CO2, yet growing seaweed there would also take nutrients from the organisms that live there. It’s like cutting down a forest to plant something else that absorbs CO2, assuming humans can do it better than nature.’
Read more:
Klaus Hubacek analyses the effects of various green solutions to reduce CO2 emissions — such as planting more trees, sharing cars, or working less — to find out whether they realize their intended outcome. Spoiler: almost everything has a downside, yes, even planting trees in some cases.
Earth's natural carbon cycle becomes unbalanced if we, humans, continue to release extra carbon dioxide (CO2) into the atmosphere. In this overview article about the carbon cycle, you can find out how Earth generally keeps itself in balance and how we, humans, have upset this balance over the past two hundred years.
In the year 2000, Harro Meijer, Professor of Isotope Physics at the University of Groningen, set up the Lutjewad Measurement Station near Hornhuizen. There, researchers from Groningen are mapping where CO2 in the atmosphere originates and where it ends up.
More news
-
17 February 2026
The long search for new physics
-
10 February 2026
Why only a small number of planets are suitable for life
