Oligomerization and activation of GPCRs
G protein-coupled receptors (GPCRs) are proteins are crucial in eukaryotes for signal transduction (e.g. hormones and odors) and are a very common target for pharmaceutical compounds. They constitute the largest class of membrane proteins in the human genome that share a common architecture, each consisting of a single polypeptide with an extracellular N-terminus, an intracellular C-terminus and seven hydrophobic transmembrane domains linked by three extracellular loops and three intracellular loops.
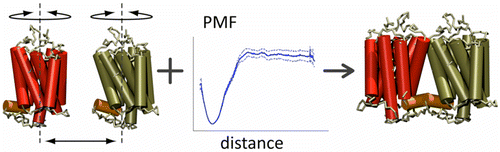
We are studying the assembly of these proteins, which is critical to their function. Whether looking at specific GPCRs [1,2] or looking at structural determinants that can govern the organisation of GPCRs in general [3], it has become clear that coarse-grain molecular dynamics can provide us with insight into their aggregaton pathway.
[1] X. Periole, T. Huber, S.J. Marrink, T.P. Sakmar. G protein-coupled receptors self-assemble in dynamics simulations of model bilayers. JACS, 129:10126-10132, 2007.
[2] A.M. Knepp, X. Periole, S.J. Marrink, T.P. Sakmar, T. Huber. Rhodopsin forms a dimer with cytoplasmic helix 8 contacts in native membranes. Biochemistry, 51:1819–21, 2012.
[3] X. Periole, A.M. Knepp, T.P. Sakmar, S.J. Marrink, T. Huber. Structural determinants of the supra-molecular organization of G protein-coupled receptors in bilayers. JACS, 134:10959–65, 2012.