Computational enzyme design
Computational enzyme design holds great promise for providing new biocatalysts for synthetic chemistry. Recent successes with de novo computational protein design and redesign of enzyme active sites suggest that computational methods can be used for controlling enzyme functionality.
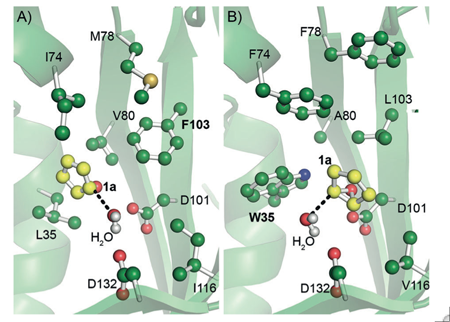
We collaborate with the Biocatalysis group of Dick Janssen to establish a high-throughput computational framework aimed at rational enzyme design. To engineer enzyme stereoselectivity, we introduced the CASCO strategy (CAtalytic Selectivity by COmputational design), and to improve thermostability, we developed the FRESCO (Framework for Rapid Enzyme Stabilization by COmputational libraries) approach. Both methods require far less screening than conventional directed evolution.
[1] H.J. Wijma, R.J. Floor, S. Bjelic, S.J. Marrink, D. Baker, D.B. Janssen. Enantioselective Enzymes by Computational Design and In Silico Screening Angew. Chem., 127:3797-3801, 2015
[2] M.M. Heberling, M.F. Masman, S. Bartsch, G.G. Wybenga, B.W. Dijkstra, S.J. Marrink, D.B. Janssen. Ironing out Their Differences: Dissecting the Structural Determinants of a Phenylalanine Aminomutase and Ammonia Lyase ACS Chem. Biol., 10:989-997, 2015
[3] H.J. Wijma, S.J. Marrink, D.B. Janssen. Computationally efficient and accurate enantioselectivity modeling by clusters of molecular dynamics simulations J. Chem. Inf. Model.,54:2079–2092, 2014
[4] R.J. Floor, H.J. Wijma, D.I. Colpa, A. Ramos-¬Silva, P.A. Jekel, W. Szymański, B.L. Feringa, S.J. Marrink, D.B. Janssen. Computational library design for increasing haloalkane dehalogenase stability ChemBioChem, 15:1660-1672, 2014
[5] H.J. Wijma, R.J. Floor, P.A. Jekel, D. Baker, S.J. Marrink, D.B. Janssen. Computationally designed libraries for rapid enzyme stabilization PEDS, 27:49-58, 2014
| Last modified: | 25 June 2015 12.52 a.m. |
