Why does it take so long to develop a treatment for COVID 19?
by: Marvin Hanisch
Due to the fast innovation cycles of many high-tech companies like Apple, Tesla, Amazon or Samsung, we are used to rapid progress. Every year, there is a new iteration of smartphones, operating systems and televisions. In contrast to this rapid innovation, it seems to take a comparatively long time to develop an effective treatment for COVID‑19, although coronaviruses have been known and studied for decades, the economic incentives for finding a treatment are strong, and the typical regulatory hurdles have been significantly reduced. So why does the development of new drugs and vaccines take so long and how can we improve the process?
Reason 1: Drug and vaccine development requires large and long-term investments
Innovation cycles in the biopharmaceutical industry are particularly long and extremely expensive. The average development time for a drug from the laboratory to approval is between 10 and 12 years, and the average cost of developing a single drug is over 1.3 billion US dollars. These costs are caused by the highly qualified personnel needed, expensive laboratory equipment, and mandatory clinical trials. In addition, there is a high risk that a drug candidate shows critical side effects or proves ineffective, with the result that only about one in eight drug candidates is ultimately approved.[1]
To ensure a continuous flow of innovation, it is particularly important to create long-term incentive systems for basic and applied research. For example, many of the clinical studies on MERS or SARS were terminated after the spread of the virus had subsided. However, precisely because it takes so long to get drugs from bench to bedside, it is paramount that the development of drugs for critical, fast-spreading diseases caused by viruses is constantly advanced. Of course, profit-driven companies will only participate in research and development if there are sufficient economic incentives to do so, which is typically not the case for potentially emerging diseases. In the absence of private investment, public actors should step in and provide incentives for continued research and development efforts, especially in the area of rapidly spreading infectious diseases.
Reason 2: Biopharmaceutical companies depend on cooperation and intellectual property protection
Due to the high complexity and costs associated with drug development, biopharmaceutical companies often depend on collaborations with other organizations for the development of new drugs. In fact, the rate of inter-organizational cooperation in the biopharmaceutical industry is one of the highest across all industries. Through collaborations, biopharmaceutical companies can quickly access new technologies from universities, innovative start-ups, and competitors, thus accelerating the process of introducing new innovations.
A major hurdle for cooperation is that the underlying technologies typically represent very valuable and highly sensitive intellectual property, making companies reluctant to share their knowledge and enter collaborations at an early stage. Before engaging in a collaboration, it is crucial for companies to obtain patent protection for the invention to ensure that the high initial investment can be recouped later. Since it is time-consuming to file patents and negotiate cooperation agreements, potential inventions can be held back for fear of knowledge misappropriation. The danger of opportunistic behavior can drastically slow down the ability to react in crises. One possible remedy would be to speed up patent application procedures and to provide an inexpensive and fast system of intellectual property protection (e.g., special arbitration courts focusing on crisis-related technologies). These measures could complement existing initiatives aimed at reducing the barriers to regulatory approval of COVID‑19 treatments.
Reason 3: Medical innovation requires extensive testing
Every medical intervention has to undergo extensive testing in the laboratory and in various clinical trials to ensure tolerability and efficacy. To shorten this mandatory and time-consuming process, many attempts to find a treatment for COVID‑19 rely on drug repurposing, i.e., the use of a drug approved for another disease. The most prominent example is Hydroxychloroquine, a drug originally developed for malaria that is now used to treat COVID‑19. Repurposing has the advantage that the groundwork has already been laid (for example, that the drug is tolerable for humans), so that investigators can concentrate on the effectiveness and dosage of the drug. Thus, drug repurposing can be a promising strategy for finding treatments.
At the same time, in the search for a quick cure, many investigators currently rush to test the same known drugs, creating the risk of conducting potentially similar parallel studies, obscuring novel treatments, and tying up resources that could be deployed more productively in other contexts. From the beginning of the crisis at the end of 2019 until June 2020, 1,827 clinical trials related to COVID-19 have been registered in the US register of clinical trials, which is more than 14 times the number of clinical trials registered for SARS, MERS and Ebola together over a period of 17 years. Given this explosion of research activities, international organizations such as the World Health Organization (WHO) and foundations play a key role in coordinating initiatives, prioritizing actions, and disseminating information. In particular, when publicly supported, these organizations can reduce redundancies, connect stakeholders, and provide a forum for researchers and health professionals to exchange ideas and best practices.
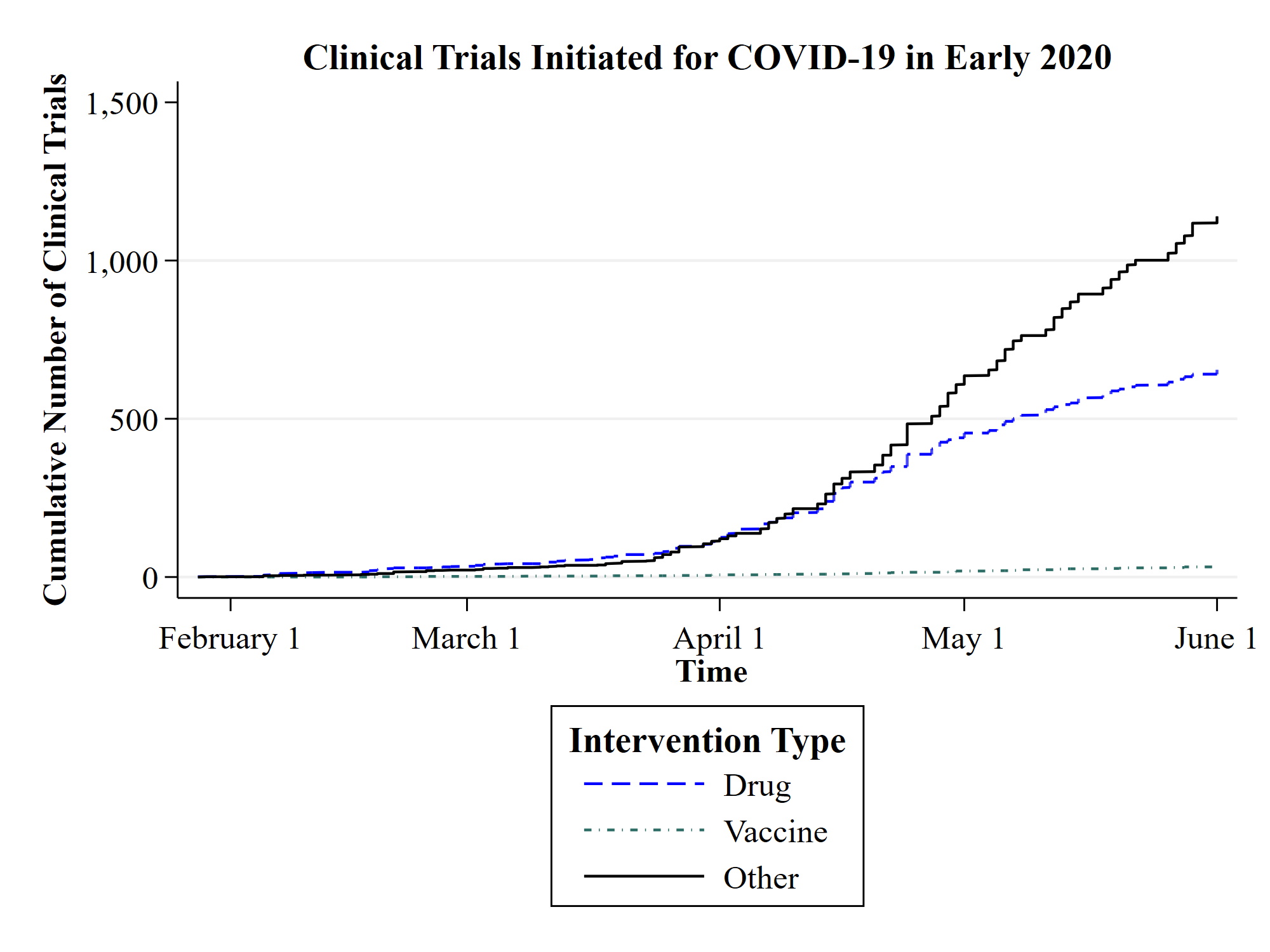
The graph shows the cumulative number of clinical trials initiated in the context of COVID-19 from 1 January 2020 to 1 June 2020. The categories “drug” and “vaccine” refer to clinical trials that test a drug or vaccine for COVID‑19. The category “other” includes clinical trials that test devices (e.g., protective gear and respiratory equipment), or provide diagnostics and prognoses. The data was obtained from ClinicalTrials.gov, the official US registry for clinical trials maintained by the US National Library of Medicine.
Learning from the past and looking ahead
Many experts believe it will take two years before we can expect a cure for COVID 19, and possibly longer if existing drugs or vaccines prove ineffective. In view of the long and expensive development cycles in the biopharmaceutical industry, we can derive three recommendations for action.
First, policy makers need to set long-term incentives to strengthen research into infectious diseases, both in basic and applied research. This will help to build a solid knowledge base and accelerate the development of new treatments.
Second, it may be helpful to establish effective and rapid intellectual property protection regimes for crisis-related technologies that complement the timely regulatory approval for necessary treatments. This way, fears of knowledge misappropriation are tamed and inter-organizational collaboration is encouraged.
Third, policy makers need to strengthen international organizations that improve the coordination of research activities in order to avoid redundancies and disseminate knowledge quickly. For example, central registers of research initiatives and platforms for regular exchange between stakeholders could bring important improvements.
Overall, despite the research initiatives that we are currently seeing, the present crisis should also be a wake-up call to rethink the existing incentive systems for drug and vaccine developments and to strengthen international cooperation again, so that we are better prepared for future crises.
[1] For details, please see: DiMasi, Joseph A., Henry G. Grabowski, and Ronald W. Hansen. “Innovation in the Pharmaceutical Industry: New Estimates of R&D Costs.” Journal of Health Economics 47 (2016): 20–33.
