Research Highlight
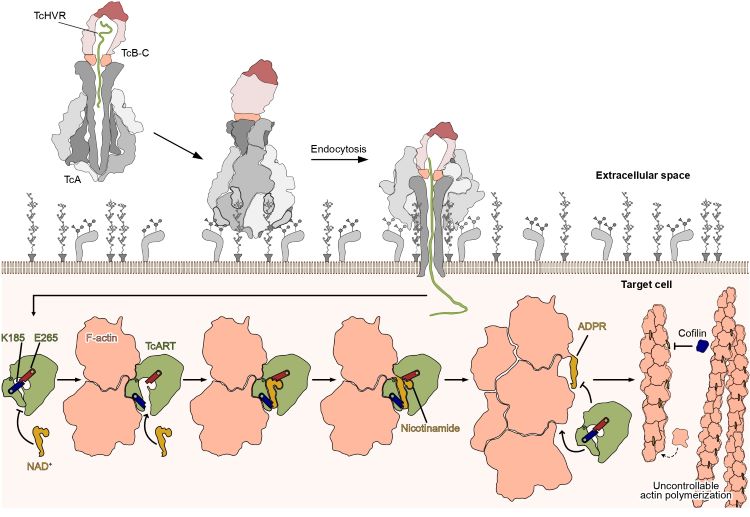
Tc toxins are potent virulence agents produced by the insecticidal bacterium Photorhabdus luminescens. These toxins comprise three distinct subunits: TcA, TcB, and TcC. The TcA subunit forms a bell-shaped assembly with a central channel surrounded by a shell with receptor-binding domains. TcB and TcC form a cocoon to encapsulate the cytotoxic enzyme termed the TcART effector. Upon entering a target cell via the TcA channel, this effector triggers lethal covalent modifications of the actin cytoskeleton of the target cell.
In this article, we demonstrated that TcART has high specificity for actin filaments and does not modify actin monomers. By determining the high-resolution cryo-EM structure of the TcART-F-actin complex, we provided the structural basis of this specificity. Further comparison of this structure with the TcART in the apo-state revealed the molecular mechanism of the covalent modification of actin in atomic detail. Finally, we determined the structure of modified actin filaments and demonstrated that the modification prevents interaction of actin filaments with actin depolymerizing factors, thus impairing actin network turnover. Collectively, our findings shed light on the intricate design and function of Tc toxins, offering a foundation for the development of novel biopesticides.
Mechanism of threonine ADP-ribosylation of F-actin by a Tc toxin.
Belyy A, Lindemann F, Roderer D, Funk J, Bardiaux B, Protze J, Bieling P, Oschkinat H, Raunser S.
Nature Communications. 2022 Jul 20;13(1):4202. doi: 10.1038/s41467-022-31836-w.
| Last modified: | 20 October 2023 4.18 p.m. |
