Smart algorithm improves diagnosis of adrenal tumours

Adrenal tumours are present in around five percent of the population and are often found incidentally when an abdominal scan is made for an unrelated reason. It is difficult to determine from the scan whether such a tumour is benign or malignant. A new method uses an algorithm that differentiates between the two tumour types based on the pattern of steroid metabolites in the patient’s urine. The method, which was published this summer, is just one of the many uses for the algorithm, says Michael Biehl, Professor of Computer Science at the University of Groningen.
The paper, which was published in July in The Lancet Diabetes & Endocrinology, has drawn considerable attention. It describes a prospective study of over 2,000 patients with an adrenal mass. ‘Benign tumours (adenomas) are fairly common, while malignant adrenocortical carcinomas are quite rare. So, it took a European collaboration to obtain enough patients,’ says Biehl. This effort was embedded in the European Network for the Study of Adrenal Tumours, ENS@T. It is a world that is far removed from the starting point of Biehl’s career. ‘I am a physicist and specialized in theoretical and statistical physics,’ he explains, ‘During the 1990s, I became involved in research into artificial neural networks, which I investigated from a theoretical point of view.’ These neural networks are computer systems that are inspired by the way neurons function in the brain. They can learn and be trained for different purposes.
Coincidence
After moving to the University of Groningen in 2003, Biehl became more interested in practical applications. Together with PhD students and collaborators, he devised a new learning system: an algorithm called ‘Generalized Matrix Learning Vector Quantization’. Whereas other machine learning approaches work in rather non-transparent ways, the GMLVQ system looks for prototypical patterns. In the case of adrenal tumours, it looks for characteristic patterns that are associated with either benign or malignant tumours. Biehl’s involvement in adrenal cancer research was a happy coincidence. When he was working in Würzburg, Germany, he met endocrinologist Wiebke Arlt in a social setting. She later moved to the University of Birmingham (UK), while Biehl transferred to Groningen. They kept in touch and collaborated on the differential diagnosis of adrenal tumours.
Finding patterns in the data
The adrenal gland produces steroid hormones and the excretion pattern can change in adrenal cancer cells. Endocrinologists measured a panel of up to 32 steroid metabolites in urine from patients and Biehl used his algorithm to find the characteristic patterns in the data. ‘The GMLVQ system defines typical examples for patterns from benign and malignant tumours,’ he explains. ‘All patients were distributed among these typical patterns and the trick is to compare the values of new patients and see in which category they fit best.’ In a retrospective study published in 2011 and based on data from 150 patients, Arlt, Biehl and their colleagues showed that they could use the algorithm to differentiate between different types of adrenal tumours. The next step was to replicate their findings in a prospective study, which meant finding a large number of new patients with an adrenal mass, who they then studied to detect the dangerous adrenocortical carcinomas.
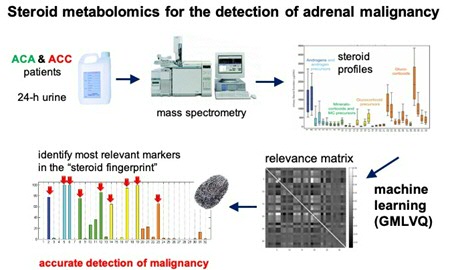
Close collaboration between medical doctors and computer scientists
‘It was a challenge to use our system on real data,’ says Biehl. The samples came from hospitals all over Europe. ‘In the early phase of the project, not all data were complete with respect to the metabolites, so we had to design a method to deal with missing data, for instance.’ The study required close collaboration between medical doctors and computer scientists. ‘It is not as if the endocrinologists sent us the data and we did our computer nerd stuff. We learned a lot from each other, on how to apply our techniques and improve the outcomes.’ PhD students in both research groups are being jointly supervised by Biehl, Arlt and Kerstin Bunte (a Rosalind Franklin Fellow at the Bernoulli Institute) in weekly meetings. The close collaboration has paid off, as demonstrated by the paper in The Lancet Diabetes & Endocrinology. By combining different markers, the differential diagnosis of adrenal masses has improved a lot. Apart from the metabolite patterns, the HU number (a measure of the opaqueness of the adrenal mass on a CT scan) and the size of the mass were used to discriminate between patients at a high, moderate or low risk of adrenocortical carcinoma. This would mean that low-risk patients no longer need to be exposed to invasive and costly follow-up diagnostics.
Included in forthcoming guidelines for clinical practice
Biehl expects that the new diagnostic method will be included in forthcoming guidelines for clinical practice. ‘There is still some work to be done to harmonize the method and to adapt it to the new ways of measuring the metabolites that are currently being introduced.’ Also, the panel of metabolites needs looking into. ‘The patterns we see in different patients tell us something about what is happening in the body of each patient,’ explains Biehl. This information can be used to better understand the disease, or to differentiate between different types of benign tumours. ‘One type, for example, is the cause of a disease called Cushing’s syndrome.’ Furthermore, it might be possible to correlate changes in the metabolite pattern during treatment for adrenocortical carcinoma to the outcome.
More applications
Apart from these medical applications, Biehl is using the algorithm in a range of other applications, such as genomics research and the classification of galaxies in astronomy. ‘We are also working with the Neuroimaging Center Groningen to diagnose degenerative neural diseases such as Alzheimer’s or Parkinson’s. There is also another collaboration with Birmingham, to differentiate joint inflammation caused by rheumatoid arthritis, based on cytokine measurements. It is fascinating to see how versatile our algorithm is.’
More information
- Michael Biehl
- Paper in The Lancet Diabetes & Endocrinology: Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study
Text: René Fransen
More news
-
29 January 2026
Microplastic research - media hype or real danger?
-
27 January 2026
ERC Proof of Concept grant for Maria Loi
