Nieuwe bindingsplaats in celmembraan voor het ‘Tubby’ eiwit
De RUG-onderzoeksgroep moleculaire dynamica onder leiding van Siewert-Jan Marrink bestudeert de wisselwerking tussen eiwitten en vetten (lipiden) in membranen via simulaties. Samen met collega’s uit Duitsland ontdekten zij een tot nu toe onbekende bindingsplek in het vat-vormige Tubby (‘mollig’) eiwit. Die kan helpen bij ons begrip van verschillende ziekten.
Als het Tubby-eiwit niet goed werkt kunnen er verstoringen ontstaan in de energiebalans van cellen, met als gevolg overgewicht, afbraak van het netvlies of gehoorverlies. Wat er precies mis gaat is nog niet duidelijk, daarom is het belangrijk meer te weten over wat Tubby-eiwitten precies doen. Er bestaat een hele familie van deze eiwitten, die allemaal een belangrijke rol hebben bij het transporteren van eiwitten binnen de celmembraan. Zij binden zich daar aan receptoreiwitten en verplaatsen zich met dat eiwit naar kleine haartjes op de celmembraan, de cilia. Die haartjes werken als een soort antennes die met behulp van de receptoreiwitten signalen oppikken uit de omgeving en doorgeven naar het binnenste van de cel. Als de functie van Tubby is verstoord, komen de receptoreiwitten niet meer aan en werken de antennes niet goed. Daardoor kunnen er ziekten ontstaan.
Mutaties
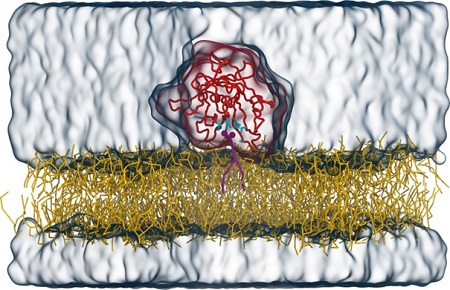
Om zijn transporttaak goed te kunnen uitvoeren moet Tubby zich vasthechten aan de binnenkant van de membraan via een specifiek lipide (met de naam PI(4,5)P2), dat alleen in de celmembraan voorkomt. Een team onder leiding van dr. Sebastian Thallmair (Frankfurt Institute for Advanced Studies), prof. dr. Dominik Oliver (Universiteit van Marburg), en RUG-hoogleraar Siewert-Jan Marrink heeft het bindingsmechanisme van Tubby aan het lipide in detail onderzocht. In een artikel dat woensdag 7 september verscheen in het tijdschrift Science Advances, beschrijven de onderzoekers de ontdekking van een nieuwe, tweede bindingsplaats voor dit lipide op het Tubby eiwit. ‘Door het gebruik van computermodellering konden we een tweede bindingsplaats vinden voor het lipide, naast de al bekende bindingsplaats’, zegt Sebastian Thallmair.
Om de resultaten van de computerberekeningen te controleren bracht het team mutaties aan in de voorspelde bindingsplaats en zagen dat dit de werking van het eiwit verstoorde. Dit bevestigde dat de tweede bindingsplaats essentieel is voor de werking van Tubby in cellen. Die bindingsplaats stelt Tubby in staat om zich te binden aan de membraan, en speelt zo een sleutelrol in de werking van het eiwit. Bovendien bleek dat de twee bindingsplaatsen elkaar versterken. ‘Daarmee zijn er, verrassend genoeg, twee gebonden lipiden, die samen meer dan twee keer het effect hebben in vergelijking met de situatie waar slechts één lipide gebonden is’, legt Thallmair uit.
Door het Tubby-eiwit te voorzien van een fluorescerend label is het ook te gebruiken om de concentratie van deze lipiden te bepalen op verschillende plekken van de membraan. ‘We zouden bijvoorbeeld graag willen weten hoe het lipide opnieuw wordt aangemaakt nadat het is afgebroken’, zegt Thallmair. ‘Het wordt logischerwijze alleen in bepaalde, specifieke delen van de celmembraan geproduceerd.’ Via het Tubby-eiwit moet het mogelijk zijn deze delen te vinden.
Referentie: Veronika Thallmair, Lea Schultz, Wencai Zhao, Siewert-Jan Marrink, Dominik Oliver, Sebastian Thallmair: Two cooperative binding sites sensitize PI(4,5)P2 recognition by the tubby domain. Science Advances 8, 7 September 2022
Tekst: Philipps-Universiteit Marburg
Meer nieuws
-
19 december 2025
Mariano Méndez ontvangt Argentijnse RAÍCES-prijs
-
18 december 2025
Waarom innoveren, en voor wie?
-
17 december 2025
Ben Feringa wint Feynmanprijs
