First results of new High Speed AFM published
The Molecular Biophysics lab of the Zernike Institute for Advanced Materials has recently installed a new High Speed Atomic Force Microscope (HS-AFM), and now the first results with this system have been published. Using HS-AFM it is possible to acquire data ~100 times faster than by traditional AFM and instead of images, movies are generated. This means that dynamic events can be studied. Dr. Sourav Maity and Prof. Wouter Roos used this strength of the system to study the dynamics of the Endosomal Sorting Complex Required for Transport (ESCRT) family of proteins. In collaboration with researchers from Grenoble they have solved the puzzle of how certain ESCRT proteins participate in membrane scission during cell-membrane remodelling. This remodelling occurs for instance during HIV budding, multivesicular endosome biogenesis, cytokinesis , etc. Their results are published in the journal Science Advances on April 10th .
ESCRT III and the AAA-ATPase Vps4 are recruited during membrane scission, but their exact functions were unknown. During HIV budding, specific ESCRT III subunits called CHMP 2A and 3 form helical filament in the budded neck. Previous studies from the Weissenhorn lab (Grenoble) showed that Vps4 complexes are capable of disassembling the filament. It remained however unclear how the ~60 nm diameter budded neck is able to constrict, finally leading to membrane scission. Using the HS-AFM, a state of art technique to directly visualize the action of proteins in real-time and with nanometer resolution, Roos and Maity were able answer this question.
‘Vps4 hexamers can remodel an ESCRT III helix in three steps: constriction, cleavage, and disassembly’ says Groningen University Professor Wouter Roos from the Molecular Biophysics lab. The Vps4 hexamers are open loops forming a corkscrew like structure. Upon binding at the inner wall of a helical ESCRT III tubule in the budded membrane neck, the Vps4 hexamer can pull the tubule inside, causing the first depletion in the neck. Next, upon ATP hydrolysis, the screw starts rotating clockwise or anti-clockwise. This rotation will then force the helical filament to deplete further forming a constriction site in the tubule. The progressing rotation of the screw will cause a permanent cleavage to the constricted site, followed by disassembly of the filament. Interestingly, depending on the number and rotational direction of these corkscrews, the constriction site can generate dome-like end-caps, resulting in an energetically favorable condition for membrane fission. These specific structures were also observed on Cryo-Electron Microscopy images, thereby supporting the dynamic HS-AFM data.
HS-AFM is a powerful tool to study dynamics of single molecules or single particles. ‘This technique is unique in its kind, using this machine one can resolve a structure at submolecular level while imaging at a speed of molecular events, even in liquid!’ says first author Dr. Maity. Apart from the novel findings on this membrane remodeling machinery, the real time imaging has proven to be a promising method to study the dynamic nature of molecules. Since it is capable to capture molecular interactions and dynamics in real-time, it is expected that this technique will soon be used in a wide set of approaches in biology, (bio)physics and (bio)chemistry.
Sourav Maity, Christophe Caillat, Nolwenn Miguet, Guidenn Sulbaran, Gregory Effantin, Guy Schoehn, Wouter H. Roos,* and Winfried Weissenhorn,*
Science Advances (2019) Vol. 5, eaau7198, DOI: 10.1126/sciadv.aau7198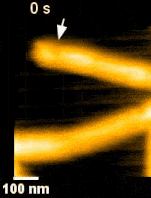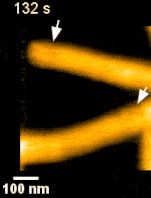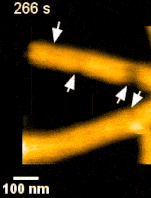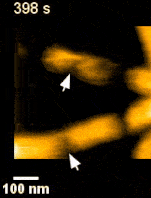
More news
-
15 September 2025
Successful visit to the UG by Rector of Institut Teknologi Bandung
