
Preclinical research on Inflammatory Bowel Disease (IBD) and fibrotic liver disease, particularly non-alcoholic fatty liver disease (NAFLD/NASH)

About Klaas Nico Faber and his research team
Since 2001, I am a principle investigator (PI) at the department of Gastroenterology and Hepatology at the University Medical Center Groningen, The Netherlands. I have broad experience in molecular and cellular biological research in bacteria, yeast, fungi and mammalian cells. My current research group consists of 2 Post-docs, 9 PhD students and 3 technicians and focusses on 1) Inflammatory Bowel Disease and 2) chronic liver disease. I closely collaborate with Gastroenterologists and Hepatologists, as well as Medical Microbiologists inside and outside our Institute, to perform translational research where patients in the end hopefully benefit from. I am passionate about studying the molecular pathways that underlie IBD and chronic (fibrotic) liver diseases and coaching my researchers to do the same. Moreover, I like technical challenges that need to be overcome to further our research field. An example of this was the development of a co-culture system where we can study the communication between human gut epithelial cells and anaerobic gut bacteria, a laboratory model that mimics the human gut. I have implemented the isolation and culture of human intestinal organoids in our laboratory primarily to incorporate the primary intestinal epithelium, either from healthy individuals or from IBD patients, in this so-called Human oxygen-Bacteria anaerobic (HoxBan) system. My ultimate aim is to develop effective therapies for patients with chronic intestinal and/or liver disease and feel that adult stem cell-derived organoids are a most suitable in vitro model to delineate disease mechanisms and to use for drug testing.

Main research lines
Molecular mechanisms underlying inflammatory Bowel Disease (IBD).
Since 2004, I am collaborating with Prof. Gerard Dijkstra, a gastroenterologist in our department, to study cell molecular mechanisms in IBD. This synergizes with research performed by the head of our department, Prof. Rinse Weersma, who focusses on -omics approaches (genomics, metagenomics, proteomics and metabolomics) to identify factors associated with IBD, making use of well-characterized patient cohorts (1000IBD) and population cohorts (LifeLines). I am particularly interested in delineating molecular mechanisms between inherited factors that predispose for IBD and how they interact with environmental factors (such as cigarette smoke), including diet and the gut microbiome. To be able to functionally analyze host-microbe interactions occurring in the human gut, we developed an in vitro system that allows co-culturing of oxygen-requiring human epithelial cells and (strict) anaerobic bacteria, the so-called Human oxygen-Bacteria anaerobic (HoxBan) system. At present, we are using patient-specific gut epithelium (generated from intestinal stem cells-derived organoids), as well as primary human intestinal fibroblasts in combination single cell RNA sequencing of intestinal tissue to study disease mechanisms and define drug targets for therapy.
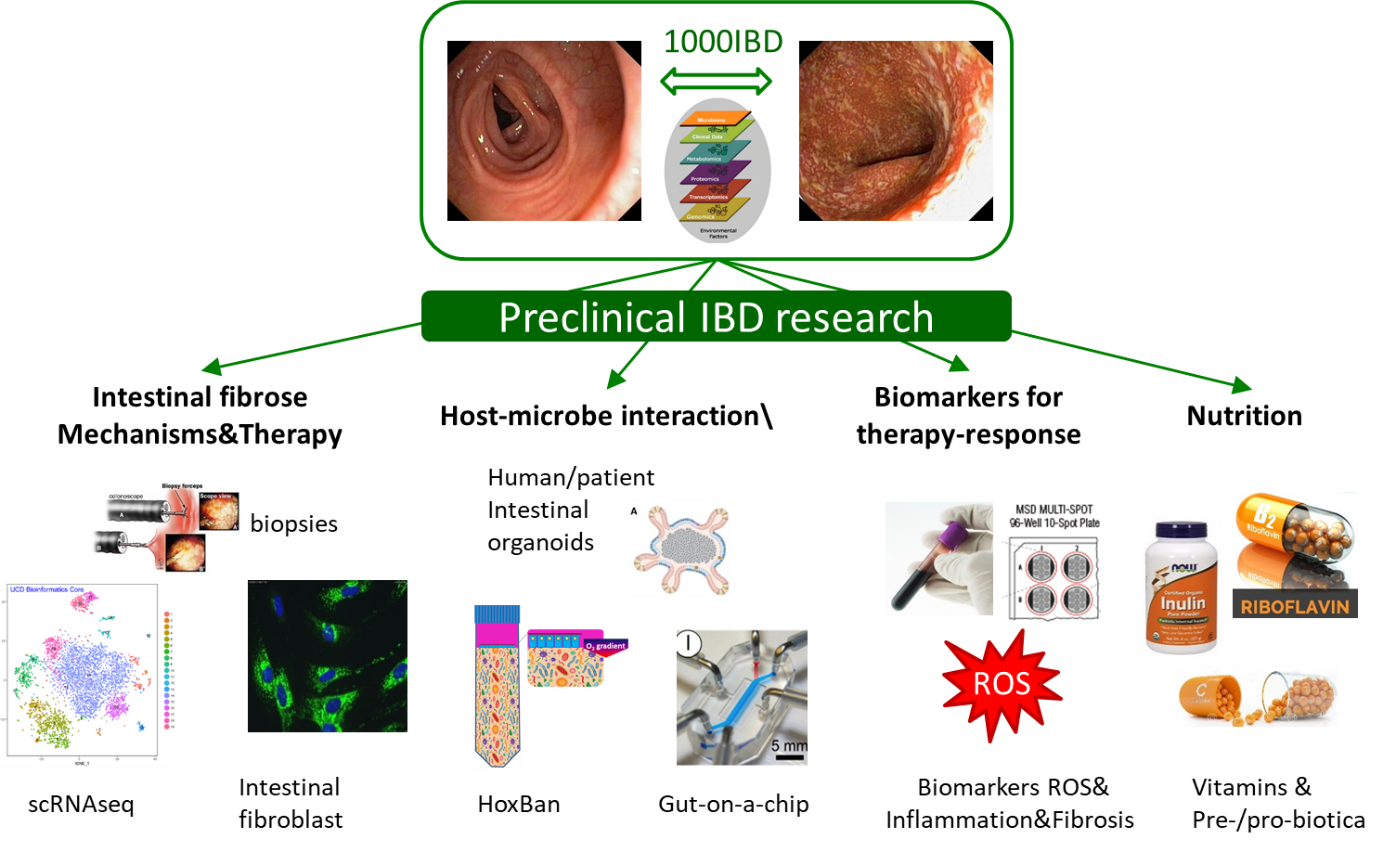
Some papers that exemplify this research line:
- Cui Y, Zhang M, Leng C, Blokzijl T, Jansen BH, Dijkstra G, Faber KN. (2020) Pirfenidone inhibits cell proliferation and collagen I production of primary human intestinal fibroblasts. Cells, in press.
- von Martels JZH, Bourgonje AR, Klaassen MAY, Alkhalifah HAA, Sadaghian Sadabad M, Vich Vila A, Gacesa R, Gabriëls RY, Steinert RE, Jansen BH, Bulthuis MLC, van Dullemen HM, Visschedijk, MC, Festen EAM, Weersma RK, de Vos P, van Goor H, Faber KN, Harmsen HJM, Dijkstra G. (2019) Riboflavin suppresses inflammation and attenuates Crohn’s disease symptoms (RISE-UP Study). J Crohn Colitis, 2019 Dec 24. pii: jjz208. doi: 10.1093/ecco-jcc/jjz208.
- Uniken Venema, WT, Voskuil MD, Vich Vila A, de Vries G, Faber KN, Dijkstra G, Xavier RJ, Wijmenga, C, Graham, DB, Weersma, RK, Festen EA (2019). Single cell RNA sequencing of blood and ileal T cells in Crohn’s disease reveals tissue-specific characteristics and potential drug targets. Gastroenterology 156(3):812-815.
- Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-mediated immune regulation in the GI-tract and its relevance for Inflammatory Bowel Diseases (Review) (2019). Frontiers in Immunology 2019 Mar 11;10:277. doi: 10.3389/fimmu.2019.00277
- von Martels JZH, Sadaghian Sadabad M, Dijkstra G, Faber KN*, Harmsen HJM*. (2017). The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. (Review). Anaerobe, 44:3-12.
- Regeling A, Imhann F, Volders HH, Blokzijl T, Bloks VW, Weersma RK, Dijkstra G, Faber KN. (2016) HSPA6 is an ulcerative colitis susceptibility factor that is induced by cigarette smoke and protects intestinal epithelial cells by stabilizing anti-apoptotic Bcl-XL. Biochim Biophys Acta-Molecular Basis of Disease 1862(4):788-796.
- Sadaghian Sadabad M, von Martels JZ, Khan MT, Blokzijl T, Paglia G, Dijkstra G, Harmsen HJ, Faber KN. (2015) A simple coculture system shows mutualism between anaerobic faecalibacteria and epithelial Caco-2 cells. Scientific Report 5:17906.
- Sadaghian Sadabad M, Regeling A, de Goffau MC, Blokzijl T, Weersma RK, Penders J, Faber KN, Harmsen HJ, Dijkstra G. (2015) The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn's disease patients. Gut 64(10):1546-52.
- Parikh K, Zhou L, Somasundaram R, Fuhler GM, Deuring JJ, Blokzijl T, Regeling A, Kuipers EJ, Weersma R, Nuij VJ, Alves M, Vogelaar L, Visser L, de Haar C, Krishnadath, van der Woude CJ, Dijkstra G, Faber KN*, Peppelenbosch MP. (2014) Suppression of p21Rac signaling and increased innate immunity mediate remission in inflammatory bowel disease. Science Translational Medicine 6(233):233ra53.
- Blokzijl H, van Steenpaal A, Vander Borght S, Bok LIH, Libbrecht L, Tamminga M, Geuken M, Roskams TAD, Dijkstra G, Moshage H, Jansen PLM, Faber KN (2008). Upregulation and cytoprotective role of epithelial Multidrug Resistance-associated Protein 1 in inflammatory bowel disease. Journal of Biological Chemistry 283:35630-35637.
Molecular mechanisms underlying (chronic) liver disease.
Since my move to the department in 2001 I have studied various aspects of liver function and liver disaease, in keeping of my strong cell biological interest. Initially, I studied the function and biogenesis of peroxisomes in the liver, in particular its role in bile acid homeostasis and cholestatic liver disease. This work has evolved in studying the role of nuclear receptors, including the farnesoid X receptor (FXR) and retinoid X receptor (RXR) in liver diseases. The main current topic is to understand the function of hepatic stellate cells in the development of liver fibrosis, and to develop innovative therapies based on 1) nutritional (a.o. vitamin A, C) 2) stem cells (including liver organoids) or 3) pharmacological compounds to treat non-alcoholic fatty liver disease (NAFLD/NASH), immune-mediated liver disease and liver fibrosis.
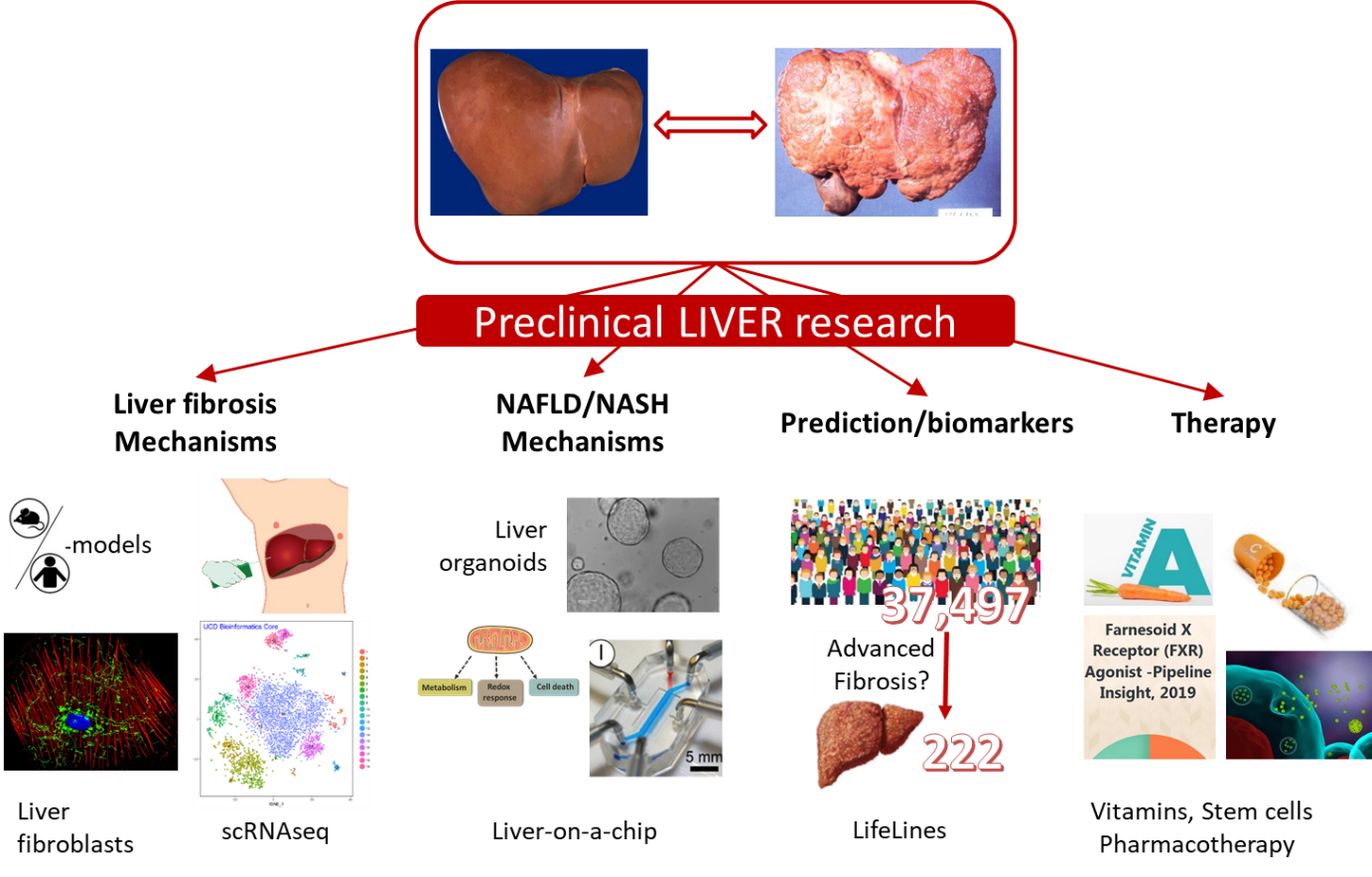
Some papers that exemplify this research line
- Saeed A, Yang J, Heegsma J, Groen AK, van Mil SWC, Paulusma CC, Zhou L, Wang B, Faber KN. (2019) Farnesoid X receptor and bile acids regulate vitamin A storage. Scientific Reports, Dec 20;9(1):19493. doi: 10.1038/s41598-019-55988-w.
- Wessel H, Saeed A, Heegsma J, Connelly MA, Faber KN, Dullaart RPF. (2019) Plasma levels of retinol binding protein 4 associate with large VLDL and small LDL particles in subjects with and without type 2 diabetes. J. Clinical Medicine 8(11). pii: E1792. doi: 10.3390/jcm8111792.
- Saeed A, Hoogerland JA, Wessel H, Heegsma J, Derks TGJ, van der Veer E, Mithieux G, Rajas F, Oosterveer MH*, Faber KN*. (2019) Glycogen storage disease type 1a is associated with disturbed vitamin A metabolism and elevated serum retinol levels. Human Molecular Genetics, 2019 Dec 9. pii: ddz283. doi: 10.1093/hmg/ddz283.
- Shajari S, Saeed A, Smith-Cortinez NF, Heegsma J, Sydor S, Faber KN. (2019). Hormone-sensitive lipase is a retinyl ester hydrolase in human and rat quiescent hepatic stellate cells. BBA-molecular and cell biology of lipids 1864(9):1258-1267.
- Saeed A, Dullaart RPF, Schreuder TCMA, Blokzijl H, Faber KN (2017) Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2017 Dec 29;10(1). pii: E29. doi: 10.3390/nu10010029. Review.
- van den Berg EH, Amini M, Schreuder TCMA, Dullaart RPF, Faber KN, Alizadeh BZ, Blokzijl H.(2017). Prevalence and determinants of non-alcoholic fatty liver disease in Lifelines: a large Dutch population cohort. PloS One 12(2):e0171502. doi: 10.1371/journal.pone.0171502
- Shajari S, Laliena A, Heegsma J, Tuñón MJ, Moshage H, Faber KN (2015) Melatonin suppresses activation of hepatic stellate cells through RORα-mediated inhibition of 5-lipoxygenase. J Pineal Res. 59(3):391-401
- Hoeke, MO, Plass JRM, Heegsma J, Geuken M, van Rijsbergen D, Baller JFW, Kuipers F, Jansen PLM Faber KN (2009). Low retinol levels differentially modulate bile salt-induced expression of human and mouse hepatic bile salt transporters. Hepatology, 49:151-150
- Pellicoro A, van den Heuvel FA, Geuken M, Moshage H, Jansen PL, Faber KN. (2007) Human and rat bile acid-CoA:amino acid N-acyltransferase are liver-specific peroxisomal enzymes: implications for intracellular bile salt transport. Hepatology. 45:340-348.
Complete List of Published Work in MyBibliography: https://www.ncbi.nlm.nih.gov/pubmed/?term=Faber-kn
