Proces in Parkinson-eiwit wellicht toekomstige therapeutische piste

Een internationale groep onderzoekers, geleid door Wim Versées van de Vrije Universiteit Brussel en in nauwe samenwerking met Arjan Kortholt van de Rijksuniversiteit Groningen, heeft een essentieel mechanisme in het ‘Parkinson-eiwit’ LRRK2 ontrafeld. Uit hun studie blijkt een directe link tussen de zogeheten dimerisatie van het eiwit – twee kopieën die samengebonden zijn – en mutaties die leiden tot Parkinson. Dit proces kan op termijn een interessante therapeutische piste vormen om de ziekte te bestrijden. Het onderzoek is op 18 oktober 2017 gepubliceerd in het toonaangevende vakblad Nature Communications.
Wereldwijd lijden zo’n vier miljoen mensen aan de ziekte van Parkinson. En door de vergrijzing ziet het ernaar uit dat het probleem nog zal toenemen. De meest frequente genetische oorzaken zijn mutaties in LRRK2, dat onder meer een ‘kinase’ en een ‘GTPase’ (twee soorten enzymen) bevat. Omdat dit kinase aan de basis ligt van problemen in de neuronen, werden kinaseremmers al klinisch getest. Die veroorzaken op termijn echter long- en nierproblemen. Wetenschappers zoeken daarom naar alternatieven om LRRK2 aan te pakken.
Parkinson-eiwit
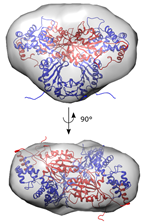
In nauwe samenwerking met Arjan Kortholt (GBB-RUG) ging het team van Wim Versées (VIB-VUB) aan de slag om de complexe structuur van LRRK2 beter te begrijpen. Dat het kinasedeel van het eiwit actief is in een dubbele of ‘dimerische staat’, waarbij twee kopieën ervan samengebonden zijn, was al bekend. Het team onderzocht daarom hoe die verbinding precies tot stand komt. Hiervoor maakten de wetenschappers gebruik van verwante eiwitten die voorkomen in bepaalde bacteriën.
Nieuw doelwit
Versées: “Het GTPase-enzym, een onderdeel van LRRK2 zélf, reguleert de toestand van het eiwit. Het bepaalt dus of het LRRK2 in zijn inactieve ‘enkele’ staat of actieve ‘dubbele’ staat voorkomt. Er is ook een duidelijke link tussen de dimerisatie en genetische mutaties als oorzaak van Parkinson. Dit proces vormt dus een aantrekkelijk nieuw doelwit voor toekomstige ontwikkeling van medicijnen.”
Mijlpaal
Kortholt: “Onze studie vormt een mijlpaal in de langlopende wetenschappelijke discussie over de dimerische staat van LRRK2 en de link met Parkinson. Maar hoewel dit een grote stap voorwaarts is, zal het nog lang duren eer we Parkinson tot in de details begrijpen en kunnen behandelen.”
Meer informatie
Prof.dr. Arjan Kortholt, Celbiochemie - Groningen Biomolecular Sciences and Biotechnology Institute (GBB), Rijksuniversiteit Groningen of prof.dr.ir Wim Versées, VIB-VUB Center for Structural Biology, Vrije Universiteit Brussel.
A homologue of the Parkinson’s disease-associated protein LRRK2 undergoes a monomer-dimer transition during GTP turnover, Nature Communications, 18 oktober 2017; DOI 10.1038/s41467-017-01103-4.
Meer nieuws
-
17 februari 2026
De lange zoektocht naar nieuwe fysica
-
10 februari 2026
Waarom slechts een klein aantal planeten geschikt is voor leven