Onderzoekers onthullen hoe vetachtige moleculen van tuberculose-bacterie de afweer prikkelen
Een internationaal team van wetenschappers heeft een methode ontwikkeld om duizenden vetachtige moleculen te identificeren die effect hebben op het menselijke afweersysteem. Deze informatie helpt bij het ontwikkelen van vaccins of behandelingen tegen tuberculose.
FSE Science Newsroom | René Fransen en Harvard University
Het onderzoek is uitgevoerd onder leiding van wetenschappers van Brigham Women’s Hospital (Boston, VS) en Monash University (Australië). Ook de groep van RUG-hoogleraar organische chemie Adri Minnaard speelde een rol in dit onderzoek, dat op 18 september is gepubliceerd in het wetenschappelijke tijdschrift Cell.
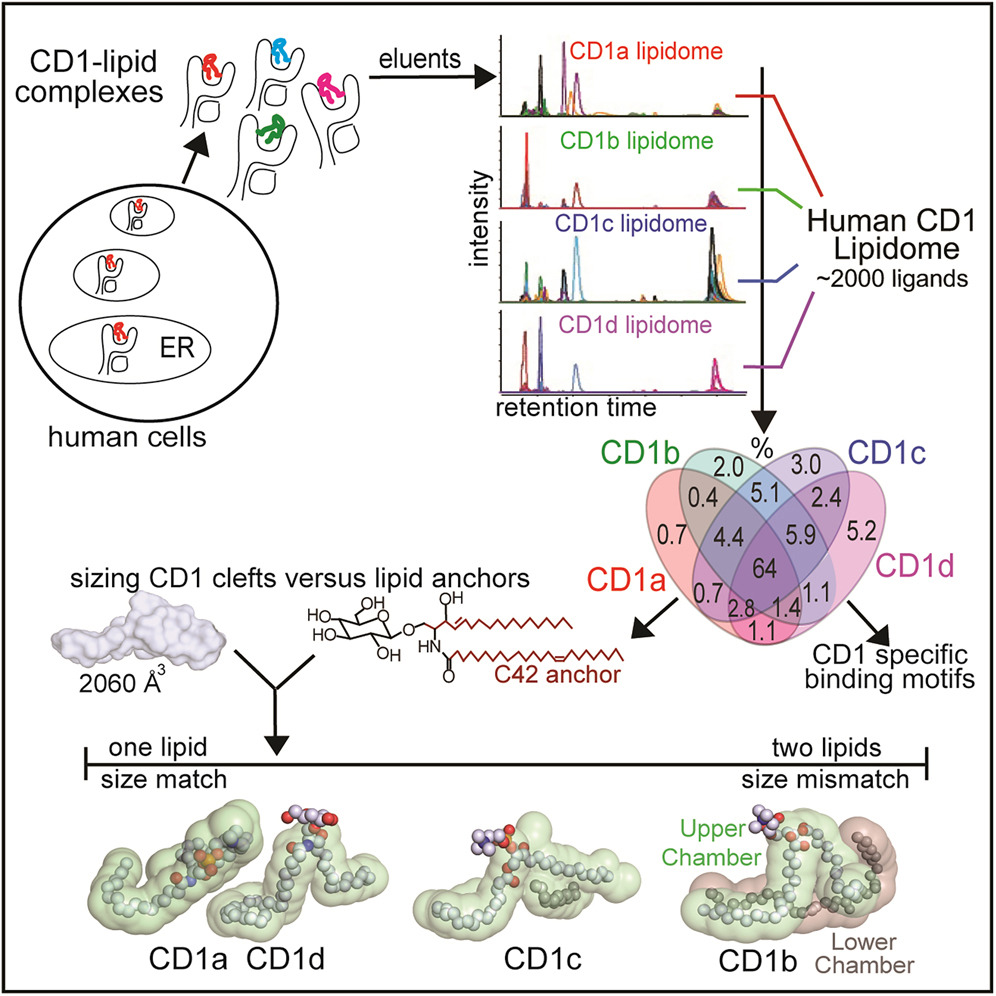
Tot in de jaren 1990 dachten immunologen dat alleen eiwitten het afweersysteem konden activeren. Maar toen werd ontdekt dat lipiden (vetachtige stoffen) kunnen worden gevangen door gespecialiseerde moleculen die ze presenteren aan het afweersysteem, zodat er een afweerreactie ontstaat. In de studie die nu is verschenen in Cell beschrijft het team de ontwikkeling van een gevoelige methode waarmee ze meer dan tweeduizend lipiden identificeerden die gebonden zijn aan zogeheten ‘CD1 antigeen presenterende moleculen’. Deze moleculen zorgen dat het afweersysteem de lipiden kan herkennen.
(lees door onder de illustratie)
Proces van jaren
De functie en positie van lipiden in de cel is totaal verschillende van die van eiwitten. Hun structuur is bijvoorbeeld niet gecodeerd in genen. Het vermogen om een heleboel lipiden die het afweersysteem prikkelen (antigenen) te meten is belangrijk voor toekomstig onderzoek. Het stelt wetenschappers in staat om een verband te leggen tussen lipiden die met ziekten zijn geassocieerd en de lijst van lipide-antigenen die nu is opgesteld.
Het Minnaard-lab is gespecialiseerd in de synthese van de complexe lipiden
Zo is nu duidelijk dat Mycobacterium tuberculosis, de bacterie die TB veroorzaakt, een groot aantal lipiden maakt die zich binden aan CD1 antigeen presenterende moleculen. Voor dit onderzoek zijn deze lipiden langs chemische weg gemaakt door de groep van Minnaard. ‘Het is om een aantal redenen erg lastig om lipiden op te zuiveren uit deze bacterie’, legt Minnaard uit. Daarom was het nodig ze zelf te synthetiseren. Dit is een complex proces, dat voor ieder afzonderlijk molecuul jaren van ontwikkeling kan kosten. Het Minnaard-lab is gespecialiseerd in de synthese van de complexe lipiden uit de M. tuberculosis bacterie.
Afweerreactie
Voor het onderzoek was ook werk op het terrein van de structuur-biologie nodig, dat is uitgevoerd in het lab van Jamie Rossjohn (Monash University). Daar is aangetoond hoe lipiden in eiwitten passen, waarbij de grootte belangrijk is. De gecombineerde structuur van eiwit en lipide laat zien welke regels er zijn voor de grootte, vorm en chemische samenstelling van lipiden die kunnen binden met CD1 en zo een afweerreactie van T-cellen veroorzaken. Die T-cellen kunnen de afweer activeren of juist remmen. Dit onderzoek is het meest recente in een reeks die in de jaren 1990 begon, toen onderzoekers van Brigham ontdekten dat T-cellen ook lipide-antigenen kunnen herkennen.
(lees door onder de illustratie)

Vaccin
‘Ik ben blij met het doorzettingsvermogen van de onderzoekers die de technologieën ontwikkelden om dit complexe systeem te creëren waarmee we grote aantallen lipiden kunnen identificeren, hun individuele structuur kunnen ophelderen om ze vervolgens in groepen in te delen’, aldus D. Branch Moody van Brigham. ‘Bij deze multidisciplinaire inspanning zijn biofysische en biologische technieken gebruikt voor de analyse van de lipiden-chemie. De lipiden voorspellen de immunologische reactie en de manier waarop lipiden worden herkend is met behulp van röntgendiffractie aangetoond.’
Het is belangrijk om te weten hoe lipiden van de TB-bacterie het afweersysteem activeren, legt Minnaard uit: ‘Met een vaccin wil je gericht de afweercellen stimuleren die reageren op de lipiden van de bacterie. Nu we weten hoe de verschillende lipiden worden gepresenteerd aan het afweersysteem kunnen we controleren of mogelijke vaccins op basis van lipiden de meest effectieve afweerreactie geven.’ Daarnaast zijn er ook lipiden die de afweer juist remmen. Deze vormen een interessant doelwit voor behandelingen van bijvoorbeeld de mycobacterie die lepra veroorzaakt.
oor onder de illustratie)
Referentie: Shouxiong Huang, Adam Shahine, Tan-Yun Cheng, Yi-Ling Chen, Soo Weei Ng, Rachel Farquhar, Stephanie Gras, Clare S. Hardman, John D. Altman, Nabil Tahiri, Adriaan J. Minnaard, Graham S. Ogg, Jacob A. Mayfield, Jamie Rossjohn, and D. Branch Moody: CD1 lipidomes reveal lipid-binding motifs and size-based antigen-display mechanisms . Cell, 18 september 2023
Meer nieuws
-
18 december 2025
Waarom innoveren, en voor wie?
-
17 december 2025
Ben Feringa wint Feynmanprijs
-
16 december 2025
FEBS Excellence Award 2025 for Alexander Belyy

