Evolving chemical system changes its environment
A chemical system of synthetic replicators shows the first signs of Darwinian evolution: two different replicators compete for a common building block, and which one wins depends on the environment. As the replicators can also change their environment, ecological-evolutionary dynamics ensue. This finding shows that Darwinian principles extend beyond biology to synthetic systems. These results, which may be used to develop new catalysts, were published by chemists from the University of Groningen (the Netherlands) in the journal Nature Chemistry on 31 August.
FSE Science Newsroom | René Fransen
What is life? This question has puzzled scientists for ages. Sijbren Otto, Professor of Systems Chemistry at the University of Groningen, addresses the question by attempting to synthesize a simple form of life from scratch. He has experimented extensively with a system of monomers, that react with each other to produce rings. These, in turn, assemble into fibres. In this process, the rings are replicated and the fibres grow and divide. Previous work showed that the fibres can also perform catalysis under the influence of light, accelerating the formation of the molecules they grow from: a primitive form of metabolism.
Adapting
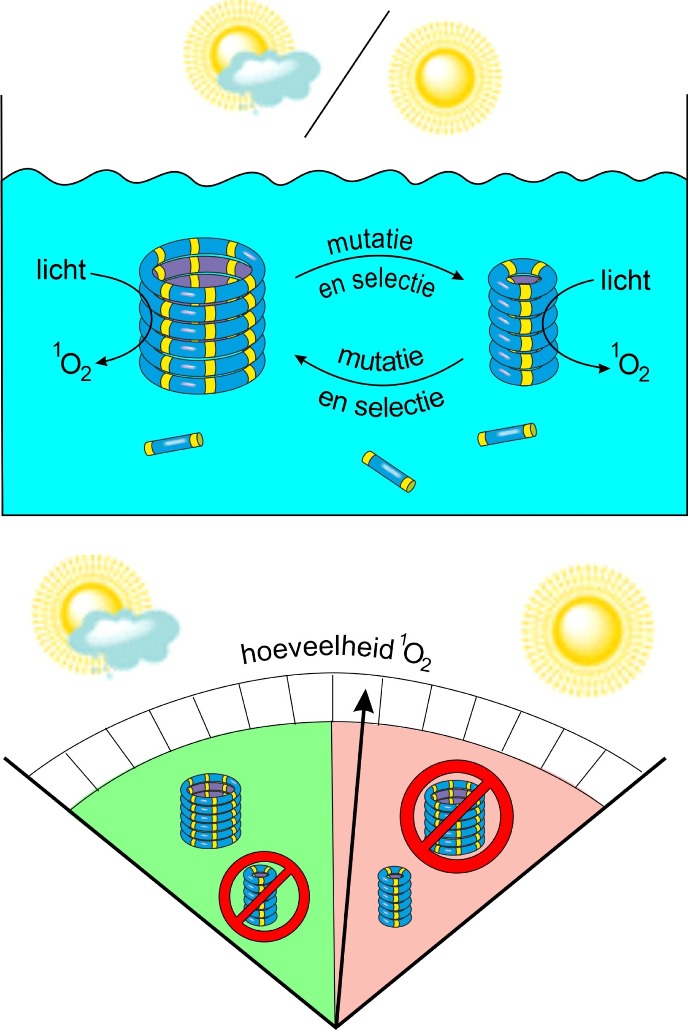
In their recent study, Otto and his team focused on another key aspect of life: Darwinian evolution. They studied fibres made from self-replicating rings of two different sizes: 3-rings and 6-rings. ‘All rings assemble from the same monomer, for which they compete’, explains Otto. ‘We then placed these systems in a flow cell while adding a solution of monomers at a constant rate. At the same time, we were removing an equal amount of fluid from the cell.’ The scientists then watched the fibres reproduce and evolve by changing their ring size.
Replicators will only survive if they can replicate faster than the rate at which they are removed by the outflow. In this system, the two replicators exhibited different growth rates in different environments: the 3-rings grew fastest if they were in a highly oxidized environment, while the 6-rings won the competition if the environment was less oxidizing. Otto: ‘We saw that the replicators could mutate into a different ring size when the oxidation state of the environment was changed. Thus, these replicators appear to be capable of adapting to a changing environment.’

Dynamics
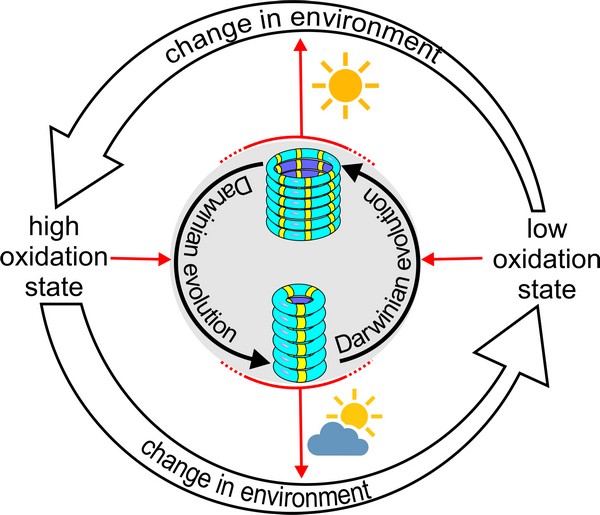
The researchers further developed this system by giving the replicators the ability to alter the oxidation state of their environment by themselves in response to light. Weak light conditions caused only little oxidation, allowing the 6-ring replicators to dominate. However, under strong light, the 6-ring replicators increased the oxidation level, thereby poisoning its own environment. This reduced the ability of this replicator to grow, and the mutant 3-ring fibres now took over.
‘Our system is very simple, yet it shows some of the dynamics normally only seen in living systems’, says Otto. ‘We showed how a kind of natural selection determines which type of replicator dominates, and also that these replicators can change their own environment, which, in turn, influences replicator evolution. Such eco-evolutionary dynamics are well known in biology, and it is now clear that they also extend to (our) synthetic system.’ But Otto does not yet call the system alive, as this would require additional features, such as the compartmentalization of the replicators in a cell-like structure.
(continue reading below the picture)
Inventive power
‘However, it is interesting that Darwinian principles, which are the cornerstone of biology, can also be introduced into our synthetic system. We have replication, a metabolism, and now also a limited kind of Darwinian evolution’, concludes Otto. ‘This is still very rudimentary, but we are keen to see if we can push our systems to become ever more life-like.’ Apart from uncovering how chemical systems can transition into living ones, such systems could also harness the inventive power of Darwinian evolution to develop novel catalysts or materials, for example.
Reference: Kai Liu, Alex Blokhuis, Chris van Ewijk, Armin Kiani, Juntian Wu, Wouter H. Roos and Sijbren Otto: Light-driven eco-evolutionary dynamics in a synthetic replicator system. Nature Chemistry, 31 August 2023
Read more about the research in the Sijbren Otto group:
08 March 2023: 2023 Izatt-Christensen Award for Prof. Sijbren Otto
26 June 2020: Life-emulating molecules show basic metabolism
19 April 2017: Evolving molecules point to principles of life
04 January 2016: The origins of abiotic species
27 March 2013: Chemical evolution
| Last modified: | 10 February 2025 09.13 a.m. |
More news
-
06 May 2025
Overcoming grid congestion: ‘Making better use of what we already have’
Grid congestion poses a major problem. There is little to no capacity to connect new households and businesses to the power grid and it risks halting the energy transition. Michele Cucuzzella, Associate Professor of Energy Systems & Nonlinear...
-
29 April 2025
Impact | Rubber recycling
In the coming weeks the nominees for the Ben Feringa Impact Award 2025 will introduce themselves and their impactful research or project. This week: Francesco Picchioni on his innovative way to recycle rubber.
-
29 April 2025
Impact | Improving Human-AI Decision-Making in healthcare
In the coming weeks the nominees for the Ben Feringa Impact Award 2025 will introduce themselves and their impactful research or project. This week: Andra Cristiana Minculescu on her research project on Human-AI Decision-Making in healthcare.
