The secrets of polydopamine coatings revealed
Dopamine is best known as a neurotransmitter. What is rather unknown, however, the underwater glue used by mussels contains large amounts of L-Dopa molecules, an analog of dopamine. Inspired by this, polydopamine coatings have recently been described that could be used to activate otherwise inactive surfaces. One problem in the development of these coatings is that their exact structure is unknown. Several models of polydopamine coatings have been put forward, but University of Groningen scientists have now shown through direct measurements what these coatings really look like. Their results were published in Nature Communications on 7 February 2023.
Polydopamine coatings show great promise: they don’t require any solvents other than water, and are biocompatible. They are versatile coatings that adhere to almost all surfaces and are useful as intermediate layers, for example on inactive surfaces like polyolefins. ‘Yet until now, we did not understand their exact composition and the way in which they form,’ explains Hamoon Hemmatpour, postdoc researcher at the Product Technology Group of the University of Groningen Engineering and Technology Institute.

Building blocks
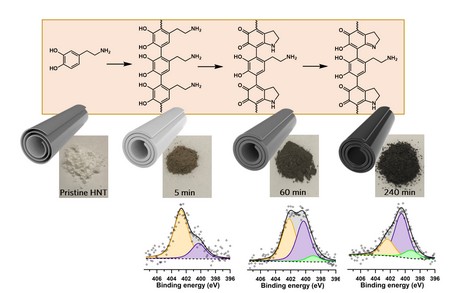
To remedy this lacuna, Hemmatpour set out to study the formation of the coatings. ‘On the macroscopic scale, this coating forms very quickly, too fast to study intermediates that might reveal what is happening,’ he explains. He therefore used nanosized tubes from a clay mineral as substrate for the coating. A large surface area and negative charges in the mineral attract intermediates from the solution, thereby slowing down the polymerization process. ‘This allowed us to take samples of the nanotubes during the polymerization process to identify the intermediates.’
Previous attempts to characterize polydopamine coatings used techniques such as mass spectrometry, which reveal the composition but not the exact chemical structure of the polymer. By studying intermediates on the nanotubes with solid state NMR and X-ray photoelectron spectroscopy (XPS), Hemmatpour could reconstruct what happens during polymerization. ‘These techniques show which chemical bonds are present.’ Once all the information was there, Hemmatpour and his colleagues could uncover the structures of the building blocks in the coatings, and thus resolve their structure.
Buffering salt
The dopamine starts polymerizing when the buffered solution reaches a pH of 8.5 or higher. ‘One thing we noticed is that the buffering salt TRIS became part of the structure, a sizeable part during the first phases of polymerization, and to a lesser degree as the reaction progressed.’ The final analysis of the process reveals auto-oxidation of dopamine, followed by crosslinking, intramolecular cyclization, and isomerization.
This study reveals the formation and structure of polydopamine coatings, which allows scientists to adapt the process for different applications. ‘We also believe that our use of clay nanotubes could be expanded to the study of other rapid processes,’ says Hemmatpour. ‘And adapting the nanotubes to increase surface area would allow us to slow down reactions even further.’
Neurons
‘There are other interesting observations from this study,’ Hemmatpour adds. ‘In neural cells, dopamine is stored in vesicles with a very low pH, but in neurodegenerative diseases, these vesicles somehow become leaky, and the dopamine accumulates in the cytosol where the pH is higher. There, the dopamine polymerizes into toxic clumps, which eventually kill neurons. Our study offers new insights into this mechanism and the intermediates of this process that can be used to find a way to treat neurodegenerative diseases.’
Reference: Hamoon Hemmatpour, Oreste De Luca, Dominic Crestani, Marc C. A. Stuart, Alessia Lasorsa, Patrick C. A. van der Wel, Katja Loos, Theodosis Giousis, Vahid Haddadi-Asl & Petra Rudolf: New insights in polydopamine formation via surface adsorption. Nature Communications, 7 February 2023.
More news
-
13 January 2026
New framework verifies AI-generated chatbot answers
