Eenvoudige reactie produceert veelzijdige bouwsteen voor biologisch actieve moleculen
Chemici van de RUG hebben een eenvoudige manier ontdekt om zogeheten chirale Z-alkenen te maken, een type molecuul waarvoor eerder geen bruikbare syntheseroute was en waarmee het veel eenvoudiger is om biologisch actieve moleculen te maken. In plaats van acht tot tien stappen verloopt de synthese nu in drie, zonder de noodzaak tussentijds producten te isoleren en te zuiveren. De sleutel was een fosfine-molecuul dat tot nu toe alleen gebruikt is om metaalhoudende katalysatoren te maken. De resultaten zijn op 13 januari gepubliceerd in het tijdschrift Science Advances.
Organische moleculen zijn zeer veelzijdig. De vele koolstofatomen die ze bevatten kunnen verbonden zijn met enkele, dubbele of driedubbele bindingen. Bovendien hebben veel biologisch belangrijke moleculen chirale centra, delen van het molecuul die in twee spiegelbeeldige varianten kunnen bestaan, vergelijkbaar met een linker- en rechterhand. Veel belangrijke organische moleculen hebben een dubbel binding, een chiraal centrum en een reactieve groep die chemische modificaties mogelijk maakt. Maar voor chemici zijn die moleculen zeer lastig te maken.
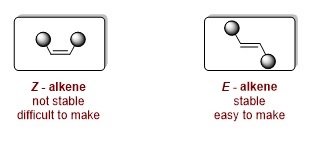
Onstabiel
Alkenen zijn verbindingen met twee koolstofatomen die via een dubbele binding aan elkaar zitten. Wanneer je dit tweetal naast elkaar afbeeldt (zie Figuur 1) zijn er twee typen te onderscheiden: Z-alkenen, waarbij de twee koolstofatomen allebei aan dezelfde kant vastzitten aan nog een koolstof (in de figuur naar boven gericht), en E-alkenen, waarbij ze de koolstof aan verschillende kanten (naar boven en naar onderen gericht) vast zit. De Z-alkenen zijn onstabiel, omdat de twee verbonden koolstofatomen aan dezelfde kant te dicht bij elkaar zitten.
‘Die configuratie vindt het molecuul niet leuk, als het de kans krijgt gaat het over in een stabieler E-alkeen. Daarom is het moeilijk om de instabiele Z-alkenen te maken’, legt Syuzanna Harutyunyan uit. Zij is hoogleraar Homogene Katalyse aan de RUG. ‘Z-alkenen zijn zeer nuttig, maar dus ook moeilijk te maken.’ Haar team wilde bovendien zo’n minder stabiele Z-alkeen maken waarbij de dubbele binding aan een chiraal centrum vastzit, met er naast een reactieve koolstof wat helemaal ingewikkeld is.

Reactiviteit
Met bekende synthetische technieken zijn er ongeveer acht tot tien afzonderlijke stappen nodig om zo’n chemische structuur te maken. Harutyunyan en haar team probeerden dit te vereenvoudigen door te beginnen met een molecuul dat fosfine heet. Co-auteur Roxana Postolache: ‘Dit molecuul wordt normaal gesproken gebruikt om metaalhoudende katalysatoren te maken. Wij ontwikkelden eerder al een manier om chiraal fosfine te maken, dat de basis vormt voor onze nieuwe synthese van Z-alkenen.’
Harutyunyan: ‘We zetten ons fosfine om in een zout. Dit zou ons in staat stellen om een dubbele binding te maken in de Z-configuratie.’ Maar het zout is erg reactief en alle pogingen de dubbele binding erin aan te brengen leverden ongewenste eindproducten op. ‘We moesten daarom een manier vinden om de reactiviteit van het zout af te stellen’, legt Postolache uit.
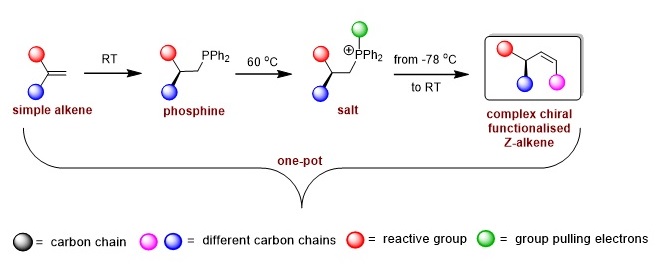
Krijtje
Voor die stap gebruikten ze allereerste een schoolbord en een krijtje. Al tekenend analyseerden ze samen de verschillende opties. Ze vonden een mogelijke oplossing voor het probleem: het toevoegen van een speciale chemische groep die een ander soort zout opleverde. Harutyunyan: ‘Dat zou elektronen weg moeten zuigen bij het fosfor-atoom van de fosfine, waardoor we de reactiviteit ervan konden aanpassen.’ Eerste auteur Luo Ge bracht dit idee van het schoolbord naar het lab: ‘We probeerden of het werkte en de eerste poging was meteen raak.’ Daarna optimaliseerden de chemici de reactie en gebruikten vervolgens deze methode om biologisch actieve verbindingen te maken.
Mogelijkheden
Een groot voordeel van de nieuwe synthetische route is dat er veel minder stappen nodig zijn, en alles in hetzelfde reactievaatje plaatsvindt. De eerste stap verloopt bij kamertemperatuur, het maken van het zout gebeurt bij milde verhitting (50 tot 70 graden Celsius) en de laatste stap, waarbij de Z-configuratie ontstaat vindt plaats bij -78 graden (zie figuur 2). Esther Sinnema, samen met Ge eerste auteur, merkt op: ‘Door fosfine te gebruiken als gereedschap voor synthese, in plaats van als katalysator, hebben we allerlei nieuwe mogelijkheden gecreëerd. We konden zo een groot aantal nieuwe chirale Z-alkenen maken en hebben daarmee bioactieve moleculen geproduceerd. In ons artikel geven we 35 verschillende voorbeelden van moleculen de we konden synthetiseren.’ Harutyunyan: ‘We verwachten dat onze nieuwe publicatie de weg zal banen voor het gebruik van commercieel verkrijgbare eenvoudige alkenen om complexe, functionele alkenen te maken via de tussenstappen van een fosfine en een zout.’
Referentie: Luo Ge, Esther G. Sinnema, Juana M. Pérez, Roxana Postolache, Marta Castiñeira Reis en Syuzanna R. Harutyunyan: Z-Selective synthesis of chiral alkenes by formal Wittig olefination of terminal alkenes. Science Advances, 13 january 2023
Meer nieuws
-
17 februari 2026
De lange zoektocht naar nieuwe fysica
-
10 februari 2026
Waarom slechts een klein aantal planeten geschikt is voor leven

