New molecular motor runs on chemical fuel
A team led by Ben Feringa, Professor of Organic Chemistry at the University of Groningen, and Depeng Zhao of Sun Yat-Sen University (Guangzhou, China) has created a unidirectional molecular motor that runs on chemical fuel, just like molecular motor complexes in our cells. One rotation of the motor molecule is completed in six steps. The newly designed molecular motor was presented in the scientific journal Nature on 6 July and could eventually be used to perform mechanical functions.
Chirality
Molecular rotary motors were first described by Feringa in 1999. This discovery earned him a share in the 2016 Nobel prize for Chemistry. His motor molecules were mainly powered by light. However, naturally occurring molecular motors, like the one powering the bacterial flagella or the ATP synthase complex, use chemical energy. So far, no artificial unidirectional rotary molecular motors operating without random Brownian motion have been created using this type of energy.
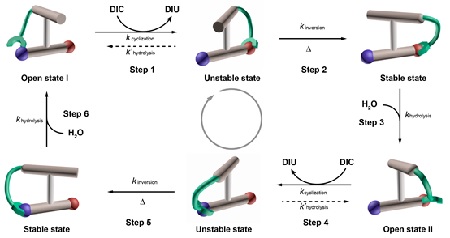
Feringa has co-led a team from the Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery at Sun Yat-Sen University, Guangzhou (China) to create just such a motor. The team’s aim was to produce a molecule that would operate autonomously and provide unidirectional rotary movement. The result is called Motor-3, a molecular motor which has two parts: a stator and a rotor, with two chiral centres on the latter. These are molecular configurations that can occur in two mirror-image versions, like a left and a right hand. Chirality is one of the keys in producing unidirectional movement.

The two parts of the motor molecule are connected by an axis that is formed by a carbon-carbon bond. Energy for the rotation is provided by a carbodiimide. Motor-3 can harvest the energy by converting this carbodiimide to urea. It can use up to 98 percent of this fuel at the early stage of the first step, while rotation is 99 percent unidirectional. The rotation speed is as yet far from that of light-driven molecular motors (which operate on a nanosecond scale), but the team is confident that by improving the design, future versions of Motor-3 will achieve similar speeds.
Motor-3 can run in two modes: synchronized motion with pulses of chemical fuel and acid-base oscillations and autonomous motion under slightly basic conditions. A motor running on chemical fuel would allow different applications, in analogy with the molecular motors that power living organisms.
Reference: Ke Mo, Yu Zhang, Zheng Dong, Yuhang Yang, Xiaoqiang Ma, Ben L. Feringa & Depeng Zhao: Intrinsically unidirectional chemically fuelled rotary molecular motors. Nature, 6 July 2022

| Last modified: | 10 February 2025 09.07 a.m. |
More news
-
06 May 2025
Overcoming grid congestion: ‘Making better use of what we already have’
Grid congestion poses a major problem. There is little to no capacity to connect new households and businesses to the power grid and it risks halting the energy transition. Michele Cucuzzella, Associate Professor of Energy Systems & Nonlinear...
-
29 April 2025
Impact | Rubber recycling
In the coming weeks the nominees for the Ben Feringa Impact Award 2025 will introduce themselves and their impactful research or project. This week: Francesco Picchioni on his innovative way to recycle rubber.
-
29 April 2025
Impact | Improving Human-AI Decision-Making in healthcare
In the coming weeks the nominees for the Ben Feringa Impact Award 2025 will introduce themselves and their impactful research or project. This week: Andra Cristiana Minculescu on her research project on Human-AI Decision-Making in healthcare.

