Hyperbranched Structures by Combined Biocatalysis

| J.van.der.Vlist at rug.nl | |
| Phone | +31 503636651 |
| Room number | 5118.0354a |
Introduction
Oligo- and polysaccharides are important macromolecules in living systems, showing their multifunctional characteristics in the construction of cell walls, energy storage, cell recognition and their immune response.
Starch is the most abundant storage reserve carbohydrate in plants. It is found in many different plant organs, including seeds, fruits, tubers and roots, where it is used as a source of energy during periods of dormancy and regrowth.
Starch granules are composed of two types of alpha-glucan, amylose and amylopectin, which represent approximately 98–99% of the dry weight. The ratio of the two polysaccharides varies according to the botanical origin of the starch. Amylose is a linear molecule in which the glucose units are joined via α-(1→4) glucosyl linkages. Amylopectin is a branched molecule in which about 5% of the glucose units are joined by α-(1→6) glucosyl linkages.
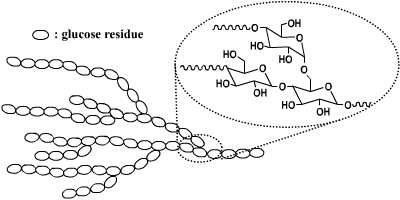
The branched structure, high amount of functional groups, biocompatibility of these structures make these architectures suitable for applications in the biomedical field and in the food industry.
Construction of well-defined carbohydrate polymer backbones is extremely difficult, as perfect control of the regio and stereochemistry of the glycosylating process in “traditional” glycosylation reactions still remains a difficult and challenging task. Most synthetic approaches are therefore based on the modification or degradation of naturally occurring polysaccharides resulting in less then perfect products.
In this respect enzymes have several remarkable catalytic properties compared with other types of catalysts in terms of their selectivity, high catalytic activity, lack of undesirable side reactions and operation under mild conditions.
The exact way of starch biosynthesis in plants is still not known today. Here we are using a tandem reaction of two enzymes to synthesize “artifical” starch or rather (hyper)branched amylose in vitro. One enzyme is responsible for building the linear (amylose) part while the other enzyme introduces the branches, phosphorylase and glycogen branching enzyme respectively.
Approach
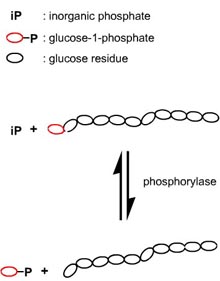
A very elegant way to synthesize well-defined amylose units is the utilization of the enzyme phosphorylase. This polymerization is the reverse reaction of the natural reaction of phosphorylases, as in vivo they phosphorolytically remove single glucose residues from a ‑(1→4) linkages within the glycogen molecules, the product being glucose-1-phosphate.
Phosphorylase can be easily isolated from potatoes and, after purification, used to catalyze the polymerization of glucose-1-phosphate in order to obtain linear polysaccharide chains with a ‑(1→4) glycosidic linkages.
The kinetics of phosphorolytic synthesis are analogous to those of living anionic polymerization so that the resulting product is highly defined with a narrow molecular weight (Poisson) distribution.
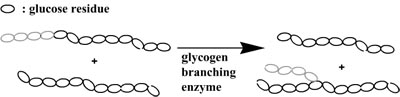
By combining both enzymes it is possible to synthesize branched structures via an one-pot synthesis as phosphorylase will polymerize linear amylose and the glycogen branching enzyme will introduce the branching points.
Both described enzymes are isolated from natural sources. Phosphorylase is isolated from potatoes whereas the glycogen branching enzyme is produced by various bacterial sources. Depending on the source the properties of the products and the reaction conditions may differ.
It was already shown that hybrid block copolymers like amylose-block-polystyrene, comb type copolymers like amylose-polysiloxane as well as amylose brushes on spherical and planar surfaces can be synthesized by covalent attachment of primer recognition units for phosphorylase and subsequent enzymatic grafting from polymerization. The resulting copolymers showed a very interesting aggregation behavior in solution, whereas the polymer brush materials demonstrated potential as column materials for enantiomeric separation.
With the described tandem reaction of two enzymes we are currently synthesizing hybrid copolymeric materials bearing (hyper)branched polysaccharide structures.
