The art of vacancies in semiconductors
Like the quotes says, no one is perfect in the world. This is also a universal principle in the field of materials science. There is no such perfect crystals in nature in spite of most used solid state materials in practical are following perfect crystalline arrangement to achieve the minimum system energy. Among them, vacancies are the most commonly seen type of defects. This is because most crystal synthesis strategies involve the high temperature, resulting to a frequent and random change of atomic position and leaving behind empty lattice sites.
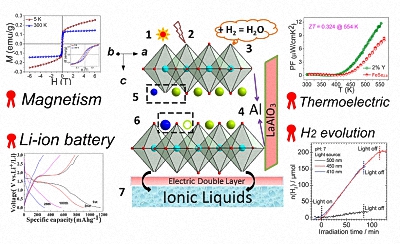
In this project, we explore the strategies to create and control various vacancies, including extrinsic and intrinsic one. By controlling their densities and distributions, it is possible to influence their intrinsic physical properties such as band-gap structures, conductivity properties, and magnetism, etc. This can further generate exciting applications in the fields of water treatment, energy storage, and physical devices such as resistance-change memory.
Publications:
- Vacancies in Materials: Perfect Imperfection, From Properties to Application. G. Li, G. R. Blake and T. T. M. Palstra, In preparation
- Band gap narrowing of SnS2 superstructures with improved hydrogen production. G. Li, R. Su, J. Rao, J. Wu, P. Rudolf, G. R. Blake, R. de Groot, F. Besenbacher, T. T. M. Palstra, J Mater. Chem. A, 2016,4(1) 209-216,
- Effect of Vacancies on Magnetism, Electrical Transport, and Thermoelectric Performance of Marcasite FeSe2− δ (δ= 0.05). G. Li, B. Zhang, J. Rao, D. H. Gonzalez, G. R Blake, R.de Groot, T. T. M . Palstra. Chem. Mater. 2015, 27 (24), 8220-8229
- High-Purity Fe3S4 greigite microcrystals for magnetic and electrochemical performance. G. Li, B. Zhang, F. Yu, A. A Novakova, M. S Krivenkov, T. Y Kiseleva, L. Chang, J. Rao, A. Polyakov, G. R Blake, R. de Groot, T. T. M. Palstra. Chem. Mater. 2014: 26 (20), 5821-5829
| Last modified: | 10 March 2016 1.58 p.m. |
