Vlijm group
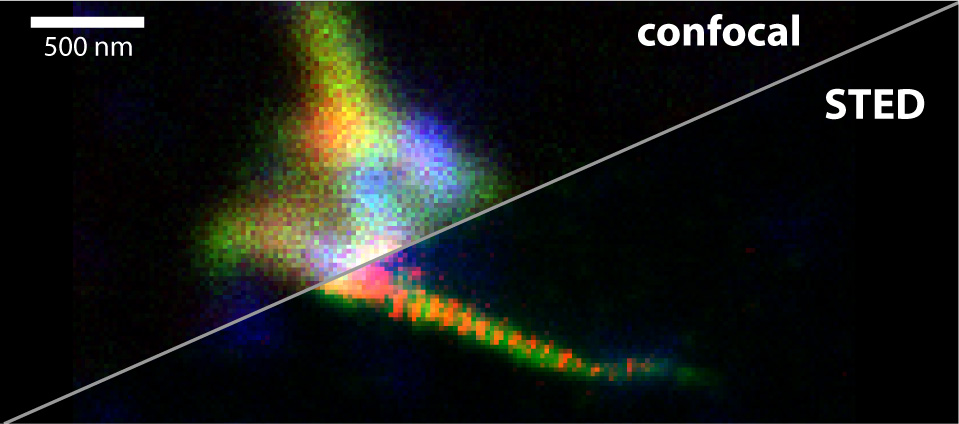
In our lab we use Stimulated Emission Depletion (STED) Microscopy to study protein structures in the cell nucleus. Our main focus is on cell division.
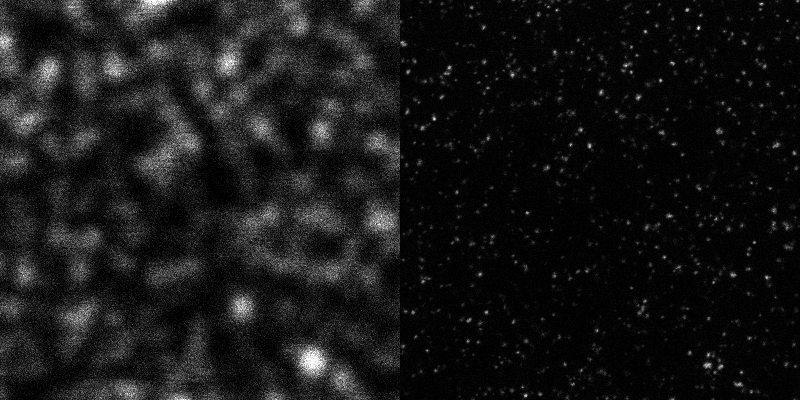
Cell division is an extremely dynamic process, and errors in this phase of the cell cycle have far-reaching consequences. Studying the protein dynamics at relevant scale is not trivial, as the dynamical interplay is at scales much smaller than the diffraction limit of light, and thus beyond the reach of traditional microscopes. Stimulated Emission Depletion (STED) microscopy on the other hand does allow imaging with a resolution of 30-40 nanometres even in cells.
The work in our group is strongly interdisciplinary, we combine optics with software developments, (live) cell sample preparation and data analysis.
We have an international and interdisciplinary research environment. For example, we work closely together both with colleagues of the Molecular Biophysics group at the Zernike Institute, the Groningen Biomolecular Sciences and Biotechnology Institute (GBB) and several international collaborators.
Stimulated Emission Depletion (STED) Microscopy
Stimulated Emission Depletion (STED) Microscopy is a special form of light microscopy where the resolution is not limited by the wavelength of light (~200nm), as postulated by the Abbe limit.

A typical resoution for STED imaging in cells today is ~30nm.
Principle
In confocal microscopy, a diffraction limited spot excites fluorophores, which emit light when they de-excite.
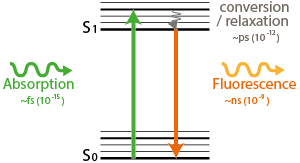
In STED microscopy, a diffraction limited spot excites fluorophores. Next, a doughnut-shaped beam stimulates most fluorophores to de-excite. Only the very center molecules are able to emit afterwards. The resolution of STED depends on the size of the dark center of the doughnut shaped beam. The higher the STED-beam intensity (Isat), the smaller the center, the better the resolution

The resolution of STED depends on the size of the dark center of the doughnut shaped beam. The higher the STED-beam intensity (Isat), the smaller the center, the better the resolution

Measurement cycle

Example