
Mission & Introduction
From inflammation to regeneration
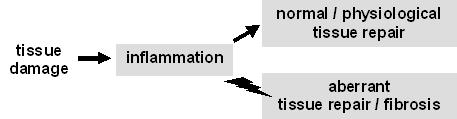
Irrespective of its cause, tissue damage invariably triggers inflammation, which, in its turn, sets the stage for tissue repair. Under normal conditions, the progression from inflammation to repair is regulated in such a way that repair is adequate, i.e. some of the structure and function of the damaged tissue is repaired. When inflammation persists and/or the transition to repair is deregulated, aberrant (e.g. exaggerated) tissue repair will lead to fibrosis and, in the long run, to organ failure.
Research within the MATRIX group addresses key cellular and molecular aspects of the interplay between inflammation, repair and fibrosis. Such aspects include cellular plasticity within the innate immune (macrophage) system, especially in relation to the instructive microenvironment, cell-cell and cell-matrix interactions, and turnover and remodeling of extracellular matrix (esp. collagen).
The many faces of inflammation
The cellular and molecular landscape of inflammatory processes varies according to their local and temporal setting (e.g. ‘where in the body’ and ‘when after damage onset’) and context (e.g. septic vs. sterile inflammation, the latter as a consequence of tissue damage or implantation of a tissue engineered construct). Our group is particularly interested in two aspects of inflammatory reactions:
- the cellular composition of inflammatory processes in settings of organ damage and in vivo foreign body reactions
- the molecular mechanisms that underlie the transition from inflammation to tissue repair.
To this end we use models of kidney injury, embryonic wound healing/closure and foreign body reactions to various types of materials.
Organ and tissue repair
Organ and tissue damage invariably leads to inflammation, which, when properly resolved, makes way for tissue repair (a.k.a. wound healing). The tight interplay between inflammatory cell subsets dictates the outcome of inflammation but is poorly understood mechanistically. Some of the research questions that trigger our interest are:
- How do macrophage subsets (M1, M2) determine the outcome of inflammation and tissue repair?
- How is the transition from inflammation to repair modulated? What are the roles of molecules such as IL-10 and Hedgehog therein?
- How can the local inflammatory microenvironment be modulated by local drug delivery, in order to achieve reparative outcomes?
- How can fetal wound healing be improved in clinical settings?
The foreign body reaction
Tissue engineering aims at the generation of functional biological tissues for the replacement of diseased or impaired tissues. To do so, often stem cells are used in combination with a biomaterial that serves as a temporary scaffold and which is gradually degraded and replaced by an autologous extracellular matrix (ECM). All biomaterials implanted into the body evoke an inflammatory response. This response by the non-specific immune system is known as the foreign body reaction (FBR). Macrophages are key players in the FBR, as well as the giant cells that are formed by means of fusion of macrophages. Controlling the activation of macrophages and the formation of giant cells are powerful tools to modulate the FBR, thereby improving regenerative strategies to repair tissues. For this we want to obtain insight in:
- The molecular basis of macrophage fusion
- The molecular basis of collagen degradation by macrophages and giant cells
- The respective role of macrophage subsets in the foreign body reaction
- The way macrophages communicate with fibroblasts and vice versa
- The role of the micro-environment in controlling phenotypic properties of macrophages
- The role of macrophages in fibrotic processes
- The role of fibroblasts in collagen deposition (capsule formation; stromal formation).
- The role of physical (surface structure, porosity, stiffness) and chemical parameters (charge, hydrophobicity, protein absorption, cross-linking, polymer chemistry, type of extracellular matrix) of biomaterials on the onset and evolution of the foreign body reaction.
Fibrosis
A pathological outcome of wound healing processes in a variety of organs (e.g. heart, liver, lung, kidney, skin) is the deposition of an excessive amount of collagen, being the hallmark of fibrosis. Fibrosis is prominent in many chronic diseases and represents an enormous health burden; it is estimated that about 40% of deaths can be attributed to fibrosing disorders. The excessive amount of collagen is mainly deposited by (myo)fibroblasts. The pathogenesis of fibrosis remains poorly understood, mainly because it is unknown what subsets of fibroblasts are involved in collagen deposition, and where these subsets originate from. In addition, we know little about the role of certain collagen-modifying enzymes in fibrosis. Understanding the pathways leading to an excessive accumulation of collagen is not only of help to define intervention points for novel therapeutics to prevent fibrosis, but will also benefit tissue engineering approaches (one of main problems in tissue engineering is the limited deposition of collagen in neoconstructs, which is the opposite of fibrosis). In our lab we investigate:
- The role of different (myo)fibroblast subsets in fibrosis as well as in fibrotic reactions seen in the foreign body reaction
- The role of lysyl hydroxylases, lysyl oxidases and other collagen-processing enzymes in the accumulation of collagen
- The role of macrophage activation in the progression of fibrosis
- The molecular basis of collagen remodelling (contraction, degradation) by (myo)fibroblasts
- Effect of tissue stiffening (due to fibrosis) on cellular processes.
Collagen and collagen receptors
Collagen is the most abundant protein in the body; it is composed of a triple helix and contains several post-translational modifications such as prolyl hydroxylation, lysyl hydroxylation and cross-linking. A correct collagen processing and turnover is pivotal in tissue repair. In tissue engineering, the main problem is the reduced amount of collagen in the neo-tissue whereas in fibrosis the opposite is seen, namely a pathological accumulation of collagen. Too much collagen hampers tissue repair due to scar formation, and too little collagen results in weakened tissue that cannot withstand the normal biomechanical forces placed upon organs.
Aside from its structural function, collagen significantly affects cell properties through cell-matrix interactions. Apart from the well-known integrins, there are several other receptors that interact with collagen, namely the discoidin domain receptors (namely DDR1 and DDR2), the mannose receptors (namely PLA2R, Endo180 and MR), and the leukocyte-associated IG-like receptor-1 (LAIR-1). One field of interest for our group is the synthesis and degradation of collagen, and the involvement of the collagen receptors Endo180 and DDR1/2 in tissue homeostasis. We are investigating the role of these relatively unknown receptors on various cell processes of fibroblasts and macrophages.
| Last modified: | 16 December 2014 3.10 p.m. |
