Radio Carbon AMS
M. Bleeker, H.A.Been and J.van der Plicht
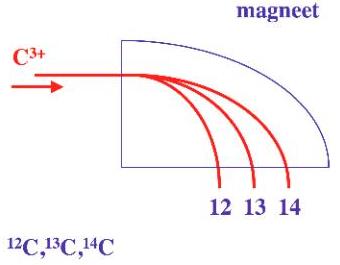
The original method to determine age from the decay of 14C atoms is accurate but requires large samples and a long measurement time, because only a small fraction of the 14C atoms decay during a realistic measurement period. A smaller sample and measurement time can be obtained when also non-decaying 14C atoms are measured. This can be achieved by separating the carbon isotopes using the difference in mass. 14C isotopes have 14 nuclei and 12C only 12. The 14C and 12C isotopes can be separated using the following physical principle: The force, needed to change the speed of an object increases with the mass of an object, or –the opposite- the change in speed decreases with mass of the object at given force. This principle is used in the mass spectrometer in the following way: atoms are first given a high speed and then the direction is changed with magnetic force. The heavier 14C atoms change less direction and are spinning out (figure 1), so the concentration of carbon isotopes can be measured at different locations.

Mass spectrometry is thus an efficient method to determine the relative concentration of 14C. However, mass spectrometry cannot distinguish between 14C and other molecules with the same mass, such as 14N, 12CH2 and 13CH. Polluting molecules are mainly removed by burning the sample to CO2 and then reducing the gas to pure C (graphite).
The graphite is pressed into a target and 58 targets can be placed on a wheel (figure 2) and then placed into the mass spectrometer (figure 3).
In the ion source a beam of Ceasium ions (Cs+) is focussed on the target. This liberates negative carbon ions (C-) from the target. A series of magnets removes non carbon ions from the beam and seperates the three carbon isotopes (12C, 13C and 14C). The chopper removes 99% of the abundant 12C, allowing a much smaller beam of carbon ions into the accelerator. The magnets between the chopper and the accelerator focus the three carbon isotopes into a single beam. The accelerator is of the tandem type, meaning that the ions are accelerated in two phases. First the negative ions are accelerated by the high voltage (+ 2.5 MV) in the center of the accelerator. By collisions with Argon atoms in the stripping canal, the C- ions are changed to C3+ ions and then triple accelerated in the second part of the accelerator. The carbon isotopes are seperated in the following 110° magnet. The abundent 12C and 13C isotopes can relatively easy be measured in cups. The 14C isotope is further purified from possible pollution using an electrostatic deflector and then the atoms are counted in an ionization chamber. Typical measurement time is 40 minutes.



| Last modified: | 18 January 2022 3.02 p.m. |
