Calibration
J. van der Plicht
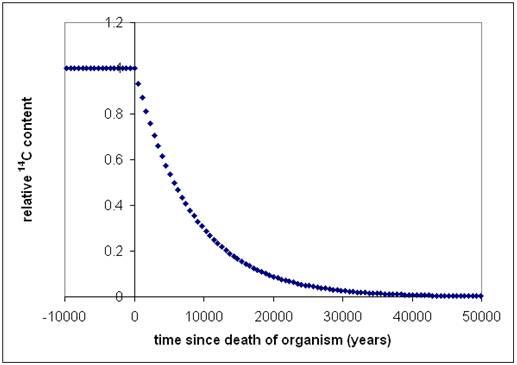
The isotope 14C is produced in the atmosphere by cosmic rays, and enters organisms because of photosynthesis by plants, and because these plants directly or indirectly serve as food for animals and humans. 14C content in the organism is in principle constant during lifetime, but decays slowly after death (figure 1). The time after death of an organism can then be determined from its relative 14C content.
Two complications arise:
1) The relative 14C content of living organisms varies between sorts of organisms.
2) Systematic deviations from the theoretical curve of figure 1 have been noticed.
Ad 1) 14C is less efficiently used during photosynthesis then 12C because of its larger mass. This effect is called ‘fractionation’ and can be corrected for by measuring the concentration of the isotope with intermediate mass: 13C. Deviations in relative 14C content also occur for organisms that live in water, because some 14C has decayed in water before it is taken up. This is called the ‘reservoir’ effect. The reservoir effect of organisms living in the upper part of the ocean and seas connected to the oceans is 400 years, so 400 years should be subtracted from the 14C age of organisms that lived in the sea. The reservoir effect is usually larger for organisms living in fresh water, caused by dissolved carbonates which are very old and therefore do not contain any 14C. The reservoir effect is even present in organisms (like people) that eat organisms (like fish) from the water!
Ad 2) 14C age of organic material appears to deviate from real age, probably because the 14C content of the atmosphere has not been constant. This problem is solved using a calibration curve. This curve is obtained by 14C dating of samples of known age.
In addition, the half-life value of 14C is not exactly known (albeit constant). Also this uncertainty is taken into account by calibration. The CIO contributes to ongoing international research to improve the calibration curve.
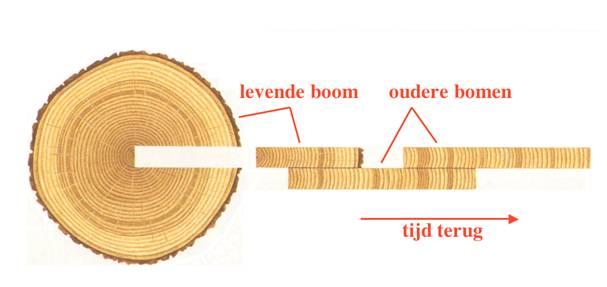
Tree rings are ideal for calibration purposes. Wood from tree rings can be used because the structure of rings depends –among others- on weather conditions during the growing season so that the ring pattern is similar for all wood grown in the same region (figure 2). Via the varying tree ring pattern, fossil trees can be linked together. This way, a tree ring chronology can be obtained. The tree ring based calibration curve now extends to ca. 12.400 years ago (figure 3).
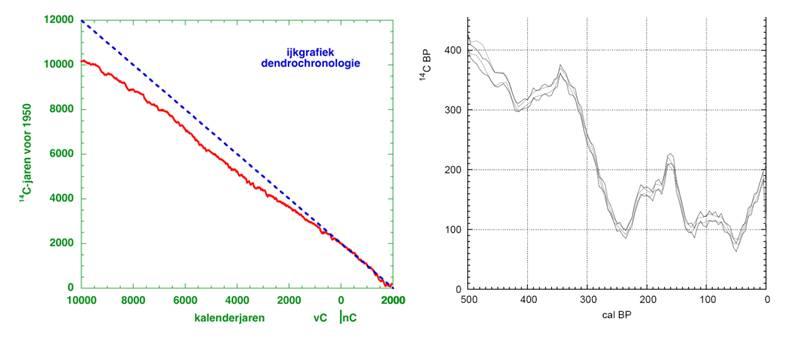
The calibration curve shows large deviations from the theoretical curve, probably because the earth magnetic field used to be weaker, enabling more cosmic rays to penetrate the atmosphere and enhancing the 14C content. The calibration curve also shows fluctuations, called ‘wiggles’ that are attributed to variations in solar activity. Wiggles complicate the determination of age, because multiple ages are sometimes related to a single 14C age (figure 4). A sample with 14C age of 150 year before present could have a real age of 20, 150, 220 or 270 year before present due to wiggles in the calibration curve, making the method less useful for the last few centuries.
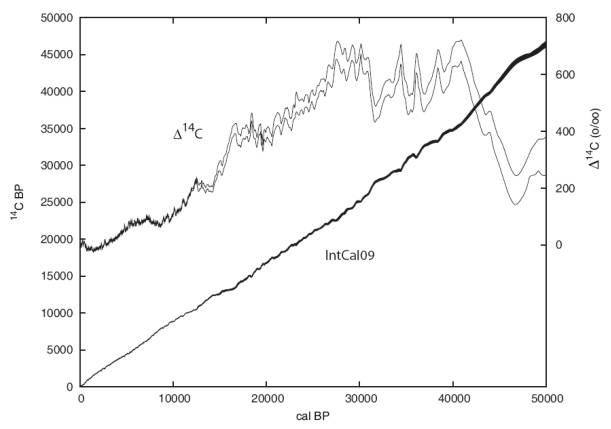
Calibration beyond 12.400 years ago depends on records other than tree rings. CIO has contributed to calibration with measurements from lake Suigetsu in Japan where organic matter in the sediment is layered and could be counted analogous to tree rings (Kitagawa and van der Plicht, 1998 ). Other calibrations compare age determination from 14C to age determination using U-series isotopes. The most recent calibration curve is extended recently back to 50.000 year before present (figure 5). The older part is mainly based on marine samples (corals and foraminifera). The age of older samples cannot be measured with the 14C method, because the signal is too small (figure 1).
References:
Kitagawa, H. and van der Plicht, J., 1998. Atmospheric radiocarbon calibration to 45,000 yr BP: late glacial fluctuations and cosmogenic isotope production. Science 279, 1187-1190.
Reimer, P.J. et al., 2009. IntCal09 and Marine09 Radiocarbon age calibration curves, 0 – 50.000 years cal BP. Radiocarbon 51-4, 1111-1150.
