Publication Science: "Cool" combination produces easier carbon bonds

By combining two century-old techniques in organic chemistry, Syuzanna Harutyunyan is able to make organic compounds with greater ease and precision. Such compounds are important for drug discovery and development. Harutyunyan’s method is described in a paper that will be published by the journal Science on 22 April.
Almost 90 percent of known active pharmaceutical ingredients contain one or more ‘heterocyclic aromatic rings’, structures that contain atoms of at least two different elements . Most of these rings contain carbon and nitrogen atoms. ‘Connecting two carbon atoms is a crucial step in synthesizing heterocycle-containing molecules’, explains University of Groningen Associate Professor of Synthetic Organic Chemistry Syuzanna Harutyunyan.
But carbon-carbon bonds are notoriously difficult to make. An intermediary step is often required, but this makes the synthetic process longer and thus less efficient. Furthermore, many pharmaceutically relevant heterocyclic molecules are chiral, which means they are present in two mirror-image versions. These versions often exhibit different biological activities. ‘So we need a way to create the right chiral version as well’, says Harutyunyan.
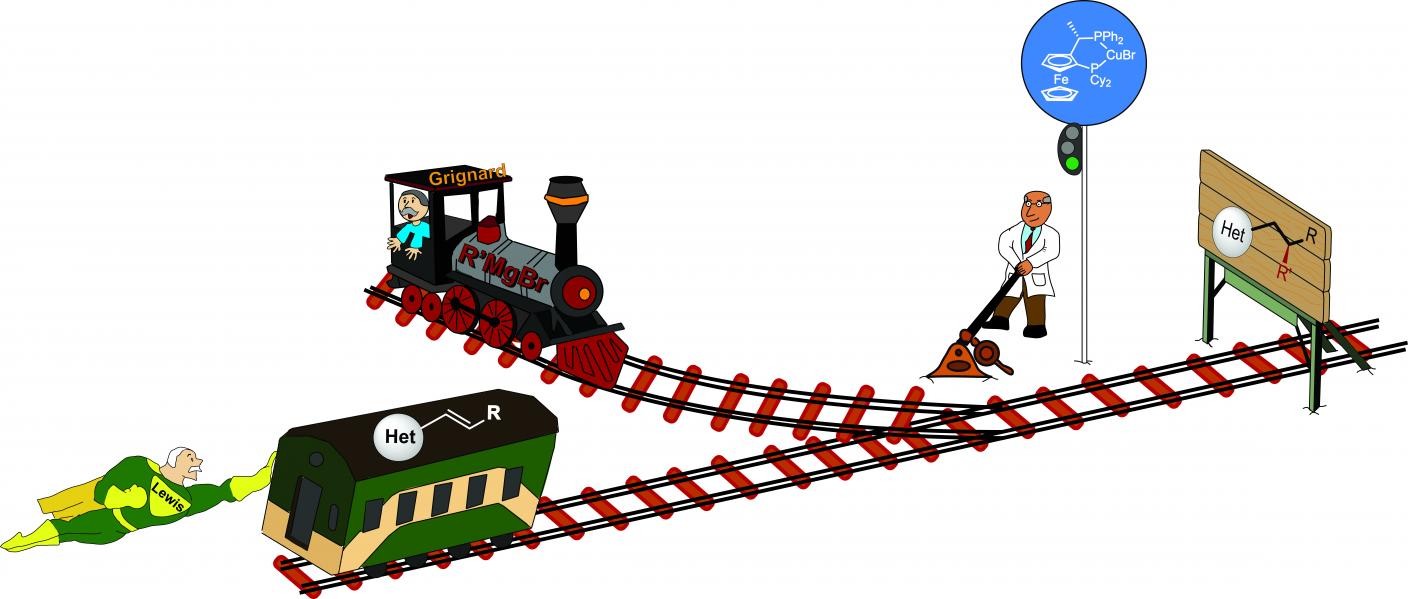
In the Science paper, Harutyunyan and her team describe just that: the efficient creation of carbon-carbon bonds with a high chiral selectivity for a wide range of nitrogen-containing heterocyclic molecules. For this breakthrough, Harutyunyan reverted to methods from the early twentieth century. Grignard reagents, originally developed by Victor Grignard, the first laureate of the Nobel Prize for Chemistry in 1912, are still an important tool in creating carbon-carbon bonds.
‘But the heterocycles didn’t respond much to the Grignard reagent, as they are very unreactive.’ This is where another pioneer in organic chemistry and a contemporary of Grignard came to the rescue: Gilbert N. Lewis, the founder of the ‘Lewis Acids/Bases Theory’. In this theory, a Lewis acid is any chemical species that attracts free electron pairs. By ‘pulling’ electrons away, the Lewis Acid can make molecules more reactive. Harutyunyan needed a very strong Lewis acid to activate the heterocyclic molecules.
Common sense says that you can’t combine the Grignard reagent and the Lewis Acid, as the Grignard reagent is a base which will react with the acid, rendering both useless. However, in previous work, Harutyunyan had shown that this was not the case at low temperatures. ‘I have spent much time studying the properties of these reagents, and that inspired me to apply them together at -78 degrees Celsius.’ Combining the Grignard reagent and Lewis Acid at a low temperature activates a carbon atom adjacent to the heterocyclic ring and produces carbon-carbon bonds. Harutyunyan added a copper catalyst to provide chiral selectivity.
A temperature of -78 degrees Celsius is a bit low for pharmaceutical companies, and the first experiments in the Groningen lab used an ether-based solvent. ‘That is too flammable for industry’, says Harutyunyan. Modifications to the process resulted in a process that works at -50 degrees Celsius in the friendlier toluene.
Pharmaceutical companies have expressed an interest in Harutyunyan’s process. ‘For drug discovery and development, you need to make variants of lead compounds. This process makes it much easier to do so.’ Meanwhile, the team is studying exactly how the Lewis Acid and the Grignard reagent work. ‘I’m a bit of a mechanistic buff. Only by finding out exactly how things work, can you improve them.’ Harutyunyan hopes to bring the reaction temperature up to -40 degrees or higher. ‘Industry is comfortable with that sort of temperature. But my main driver is that I want to understand the process, as something quite special happens.’
Reference: R.P. Jumde ; F. Lanza; M.J. Veenstra; S.R. Harutyunyan: Catalytic asymmetric addition of Grignard reagents to alkenyl-substituted aromatic N -heterocycles. Science, 22 April 2016, DOI 10.1126/science.aaf1983
Read also Bachelor’s phase research results in Science publication about Marieke Veenstra, one of the other authors.