Bill & Melinda Gates Foundation Grant for chemists in Groningen
Title: Glycolipid-based Immunization of Guinea Pigs to Activate T cells In Vivo
Participants:
· Brigham & Womens Hospital, Harvard Medical School, US, Prof. D.B. Moody
· CNRS Institute of Pharmacology & Structural Biology, Toulouse, France, Prof. M. Gilleron
· Tuberculosis Vaccin Initiative, Salisbury, UK, Prof. A. Rawkins
· University of Groningen, Stratingh Institute for Chemistry, Prof. A.J. Minnaard
Project essence. Many mycobacterial lipid antigens activate human T cells, which expand in number during human tuberculosis infections. This proposal represents the first broad effort to compare known lipid antigens for stimulation of T cell responses in vivo in guinea pigs as a new, MHC-independent approach to T cell activation and vaccination.
Vision of success. We aim to develop glycolipid antigen formulation that when injected in vivo activate strong and durable MTb glycolipid-specific T cell responses. To achieve this we will synthesize pure bioactive CD1-presented lipid antigens, including mycolyl glycolipids, mycoketide glycolipids, sulfolipids and phosphoglycolipids. Each of these lipid antigens will be combined into adjuvant formulations that activate CD1 expression on antigen presenting cells. The combined antigen and adjuvant formulations will be tested in guinea pigs for stimulation of glycolipid antigen-mediated T cell responses in vivo. Using existing tests of guinea pig immune response and newly developed guinea pig CD1 tetramers, we will compare a panel of antigens to determine which of the glycolipid antigens generate the strongest and most durable responses. Success is envisioned as detection of durable, glycolipid-specific T cell responses and providing a reliable ranking of individual antigens for the strength of immune response. Based on this information, glycolipid antigens will be formulated as vaccines that enter into studies that measure protection in models of natural TB transmission in guinea pigs, which are beyond the scope of the current three-year plan. Further, production of hundreds of milligrams of biologically active glycolipid antigens or cell surface lipids will provide a resource to support other studies to determine the targets of human B cell and immunoglobulin responses in a separately funded project.
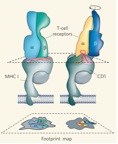
Project Purpose and Background. The human host protects itself from Mtb disease through durable antigen-specific T cell responses. In technology development, antigen-specific T cells are the key mechanism underlying vaccination, as well as the basis for widely used tests for latent TB infection, including PPD skin testing and interferon gamma release assays (IGRA). Here we propose a new core concept in which human ab T cells recognize lipid antigens bound to CD1 proteins (Fig. 1). Whereas the TB field is deeply invested in MHC-based adjuvants and peptide-specific T cells, the analogous system of CD1 and lipids is now a mature field of basic research, but has not been exploited to make better vaccines.
