Nature publication: Motion of one single transport protein observed
For the first time ever, researchers from the University of Groningen have managed to observe the motion of a single transport protein in a cell membrane. Their findings were published on 3 October 2013 in the scientific journal, Nature. Understanding how this type of protein moves while transporting molecules is an important step forwards in research into diseases.
Transporting molecules through the cell membrane is essential to life. Most molecules need a transport protein to help them pass through the fatty cell membrane. These transport proteins, which are firmly entrenched inside the cell membrane, are often highly specialized. They bond with specific molecules and transport them from outside the membrane to the inside (or vice versa). Life in the cell would grind to a halt without this transport.
The protein complex GltPh is responsible for absorbing the nutrient aspartate in microorganisms. GltPh is made up of three identical proteins, which together form one transport complex. ‘We wanted to find out exactly how the components of this complex move when transporting the aspartate into the cell’, explains Prof. Antoine van Oijen, whose group specializes in single-molecule biophysics.
Activation
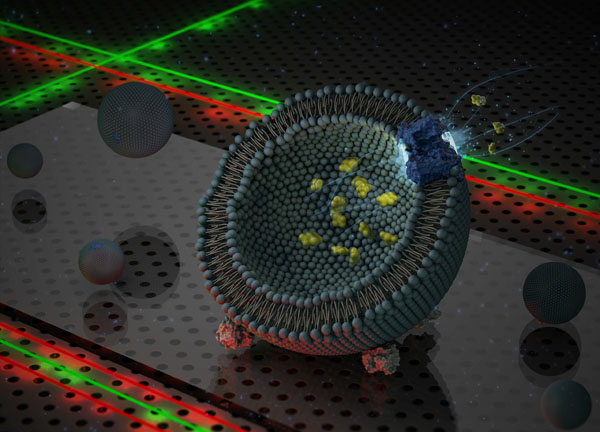
To study the transport complex, fluorescent markers were linked to two of the three proteins. The markers emitted light when illuminated with lasers. These markers influence each other’s properties: the first marker emits light, which in turn activates the second marker. The strength of the activation depends on the distance between the two markers, making it possible to monitor the distance between the two marked proteins while the aspartate is being absorbed.
Postdoctoral researcher Dr Guus Erkens carried out this technically challenging research. Erkens: ‘It was the first time that research like this has been attempted with a protein in a membrane. Fortunately, we have a lot of experience of working with membrane proteins here in Groningen.’ The research was carried out in close cooperation with with Prof. Dirk Slotboom, a specialist in membrane proteins who has spent ten years working with GltPh. Nature Structural & Molecular Biology has recently published another article on GltPh written by Slotboom.
Erkens prepared specimens with several hundred liposomes, vesicles made from fats, which form an artificial membrane that strongly resembles a natural cell membrane. By maintaining a low concentration of proteins and markers, some of the vesicles comprised just one GltPh complex with the right markers on just two proteins. These vesicles were suitable for the experiments.
Camera
‘We measure several hundred vesicles in one go and select the vesicles with just one correctly labelled complex. We then analyse the data they provide’, explains Erkens. Measurements are made using a specially adapted microscope. Lasers activate the markers and a sensitive camera records the reaction. Transport commences when aspartate and an energy source for the protein are added to the equation.
The three proteins in the transport complex form a sort of basin in the membrane, which flips over taking the aspartate inside through the membrane. ‘The question was how this happens’, says Van Oijen. ‘Do the three sub-units flip over together, one at a time, or chaotically?’ Erkens’s results show no fixed pattern in the motion. The units flip over randomly and can transport an aspartate molecule independently of each other.
The study plays an important role in medical research, as the human brain has a similar transport complex for removing glutamate, a neurotransmitter that allows nerve cells to communicate with each other. ‘If this complex doesn’t work properly, neither does the brain’, explains Van Oijen. It is possible that a defect in glutamate transport plays a role in conditions such as Alzheimer’s disease, ALS and Huntington’s disease.
Targeted drugs
‘Now that we know how the transporter is supposed to work, we will be able to search for abnormalities’, according to Van Oijen. ‘This could help us to develop more targeted drugs. However, the whole process could take another ten to twenty years.’
Van Oijen sees his research as a step in a lengthy process, which aims to study individual molecules in as natural an environment as possible. ‘Other research groups have studied GltPh in a soapy solution. This allows it to flip over, but no transport takes place as the solution does not have an ‘inside’ or an ‘outside’.’
The results of this type of research are very different from Van Oijen’s latest results. He sees this as a sign that he is on the right path. ‘It’s important to study these transport proteins in the right environment. We’ll only improve our understanding of the molecular mechanism if we can study real transport taking place through a real membrane.’ Van Oijen is now focusing on protein complexes consisting of multiple components. ‘Our ultimate aim is to find out how all these proteins work in a real cell.’
Contact details
Prof. Antoine van Oijen, tel: +31(0)50-363 9883, e-mail: a.m.van.oijen rug.nl
Prof. Dirk Slotboom, t el.: +31 (0)50-363 4187 , e -mail: d.j.slotboom rug.nl
Dr. Guus Erkens, tel.: +31 (0)50-363 8694, e-mail: g.b.erkens@rug.nl
Reference: Unsynchronised subunit motion in single trimeric sodium-coupled aspartate transporters, Guus B. Erkens, Inga Hänelt, Joris, M.H. Goudsmits, Dirk Jan Slotboom and Antoine M. van Oijen
DOI:10.1038/nature12538More news
-
17 February 2026
The long search for new physics
-
10 February 2026
Why only a small number of planets are suitable for life
