Nature Chemistry: Groningen chemists develop switchable antibiotic
Scientists at the University of Groningen have developed an antibiotic whose activity can be controlled using light. It is possible to ‘switch on’ the substance immediately before use, after which it will slowly lose its activity. Thus release of the active antibiotic into the environment might be prevented. This discovery was published on the website of the scientific journal Nature Chemistry on Sunday 15 September 2013.
Antibiotics protect us against lethal infections and are also widely used in agriculture. However, after use they end up in the environment, for example through the sewage system, where they can promote the development of resistant bacterial strains.
With this in mind, the Groningen scientists investigated whether an antibiotic could be developed whose activity could be controlled. After all, if only deactivated antibiotics enter the environment, there will be no risk of resistance.
Variants
Ben Feringa, professor of organic chemistry, is a pioneer in the field of light-activated molecular switches. His PhD student Willem Velema created variants of an antibiotic to which a photoswitchable group was attached. ‘It is difficult to predict what will happen to the activity when a group in a molecule is changed’, explains Velema. The new group will, after all, influence the effect of the antibiotic. ‘And that will have to happen in exactly the right way.’
Velema made nine variants, one of which appeared to work very well in tests that were carried out in collaboration with the research group of molecular biologist Arnold Driessen. The switch ensures that the antibiotic does not work in the normal position. However, after radiation with ultraviolet light, part of the switch molecule flips, activating the antibiotic. In chemical terms: the switchable group changes from a trans-isomer to a cis-isomer.
Half-life
In the Nature Chemistry article, the researchers demonstrate that it is possible to switch on the antibiotic at any time. Subsequently, the molecule flips back by itself from the active cis-isomer to the non-active trans-isomer. The half-life is approximately two hours at body temperature.
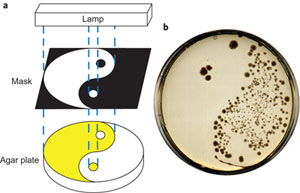
Feringa emphasizes that the experiments are only meant to demonstrate that it is possible to control the activity of an antibiotic using light. ‘The path from this idea to a working drug is very long, it could be another ten years.’ In addition to switchable antibiotics not burdening the environment, they could also be used to treat very specific areas in the body. Feringa: ‘You could take an inactive antibiotic for a skin infection and then activate it at the site where it is required. No useful bacteria in the intestines will be killed this way.’
Bacterial growth
For the time being, the switchable antibiotic is especially useful for research. ‘It is possible to inhibit the growth of bacteria very specifically at a particular site and at a specific time.’ You could, for example, investigate which factors influence the growth of the bacterium and the action of the antibiotic or the origin of resistance.
‘But this article’s most important message is that it is possible to externally control the action of a drug, composed of a relatively small molecule, in this case using light,' says Feringa. 'That is a whole new way of thinking about drug research.’
Contact:
- Prof. Ben Feringa
- Willem Velema
Referentie: Optical control of antibacterial activity. Willem A. Velema1, Jan Pieter van der Berg2, Mickel J. Hansen1, Wiktor Szymanski1, Arnold J. M. Driessen2,3 and Ben L. Feringa1,3. Nature Chemistry, online 15 September (DOI: 10.1038/nchem.1750)
- Centre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands
- Molecular Microbiology, Groningen Biomolecular Sciences and Biotechnology Institute, University of Groningen, Nijenborgh 7, 9747 AG, Groningen, The Netherlands
- Zernike Institute for Advanced Materials, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands
Financial support for this research was provided by NWO (Netherlands Organisation for Scientific Research), KNAW (Royal Dutch Academy of Arts and Sciences), ERC (European Research Council) and the Ministry of Education, Culture and Science (Gravitation programme).
More news
-
29 January 2026
Microplastic research - media hype or real danger?
-
27 January 2026
ERC Proof of Concept grant for Maria Loi
