Calorimetry Measurements
The TA Instruments – SDT 2960 offers the possibility of measuring simultaneous TGA, DTA and DSC. These types of experiments are used in determining phase transition temperatures, reaction rates, properties of volatile compounds or polymer thermal degradation profiles. They can offer qualitative and quantitative information regarding the materials physical and chemical processes. Besides this, using these measurements, the researcher can identify exothermic and endothermic transitions.
TGA (Thermo Gravimetric Analysis) measures the changes in sample mass as a function of temperature and time.
DTA (Differential Thermal Analysis) measures the difference in temperature between the sample and a reference that is thermally inert when the temperature of the system is varied. The temperature sensitivity is ΔT= 0.001°C.
DSC (Differential Scanning Calorimetry) is used to measure the heat flow associated with different changes in the material as a function of temperature and time.
The measurements can be performed in controlled atmospheres (Ar, N2, O2, air). The temperature range in which the SDT 2960 can be used is from room temperature to 1500°C (it is optimized for temperatures above 200°C, but good performance can be achieved also for lower temperatures). Typical heating rate is 5°C /min. The TGA has a sensitivity of 0.1 μg and accuracy of ±1%.
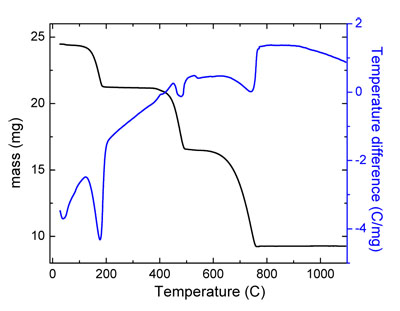
A good example of the utility of SDT 2960 in characterisation of the materials is calcium oxalate monohydrate. This has three distinct weight losses in the temperature interval 20°C - 1000°C. These transitions can be distinguished in the evolution of the mass (measured with TGA), as well as in the shape of the temperature difference profile (measured with DTA), and they correspond to the following scheme:
CaC2O4·H2O -> CaC2O4 -> CaCO3 -> CaO + CO2↑
