Peroxisome proliferation
Regulating peroxisome numbers within the cell, through synthesis and degradation events, is an essential part of cellular metabolism. In yeast, peroxisome numbers can depend on growth conditions and new peroxisomes are often produced or degraded in response to changes in carbon and/or nitrogen sources. In H. polymorpha peroxisome size and number increase by growing cells on methanol since peroxisomes house the enzymes required to utilise this carbon source. On the other hand, cells grown on glucose usually contain a single, small peroxisome since peroxisomes are not required to utilise glucose.
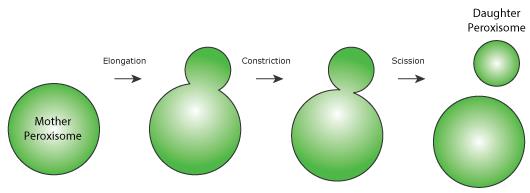
Upon induction of peroxisome proliferation, new peroxisomes can be made by the division of pre-existing organelles in a process called fission (Figure 1). Peroxisomal fission can be broken down into three steps i) elongation of the peroxisomal membrane ii) constriction of the elongation at a certain site and iii) the actual scission step, that separates the daughter peroxisome from the mother.
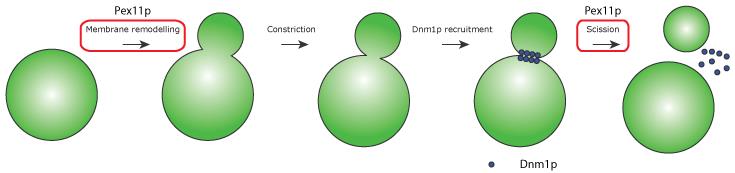
In plants, mammals and yeast both peroxisomal and mitochondrial fission requires the action of three proteins; Fis1p, Mdv1p and Dnm1p ( Schrader et al., 2015 ). However, a number of organelle-specific factors are known to regulate the individual fission processes. For example, the peroxisomal membrane protein Pex11p is involved in forming peroxisomal membrane elongations and previous data from our group demonstrated that an amphipathic helix in the N-terminal region of Pex11p controls membrane remodelling ( Opalinski et al., 2011 ). However, the role of Pex11p in peroxisome fission goes much deeper, with Pex11p also controlling the reorganisation of certain peroxisomal membrane proteins ( Cepinska et al., 2011 ), as well as the recruitment of members of the fission machinery ( Koch and Brocard, 2012 ).
The final step of peroxisome fission requires dynamin-like proteins (DLPs). These large GTPases are most likely involved in the actual organelle fission process and can be seen as molecular scissors that catalyse the separation of the mother peroxisome from the daughter.
Fis1p is a peroxisomal and mitochondrial membrane protein that was originally thought to control the recruitment of Dnm1p to the organellar membrane. However, more recent data demonstrate additional, post recruitment roles for both Fis1p and Mdv1p in mitochondrial fission.
"Recently (
Williams et al., 2015
)., we demonstrated that Pex11p stimulates the GTPase activity of Dnm1p both in yeast and mammals, establishing a vital role for Pex11p in the final step of peroxisomal fission Due to its many roles, we describe Pex11p as the
Master regulator of peroxisomal fission
(Figure 2)".
Since Pex11p plays such a central role in the peroxisomal fission process, research in our group aims to investigate the molecular mechanisms of Pex11p function. To this end, we (Dr. Chris Williams and Ann Thomas) focus on binding partners of Pex11p and employ biochemical and biophysical techniques, as well as molecular dynamics simulations, in combination with advanced microscopy and in vivo approaches, to investigate how these interactions regulate Pex11p function, as well as peroxisomal fission.
| Last modified: | 18 May 2018 4.45 p.m. |
