Climate-driven population divergence in sex-determining systems
Sex determination is a fundamental developmental process, yet its mechanisms are remarkably diverse. Vertebrates exhibit both genotypic (GSD) and temperature-dependent sex determination (TSD). The latter is particularly common in reptiles and both systems can co-occur within taxonomic families. In addition, some species show elements of both genotypic and environmental sex determination within populations. Explaining the causes behind repeated evolutionary shifts between GSD and TSD and the origin and maintenance of mixed systems are two of the greatest unsolved problems in sex determination research. The two main reasons why the diversity in reptilian sex determination has remained an enigma have been (1) a failure to empirically link incubation temperature to ecological conditions promoting TSD and (2) to establish theoretically that those conditions are sufficient to drive evolutionary shifts in sex-determining systems. In a joint theoretical and empirical effort, we set out to tackle these problems (Pen et al., Nature, 2010).
A lizard, the snow skink Niveoscincus occelatus, seemed ideally suited for our purpose. This species has populations with GSD that live in the harsh climate of the Tasmanian highlands, and populations with TSD in the more temperate coastal areas of the island. Very few species are known where GSD and TSD coexist, and even fewer with well-studied life history and ecology. Fortunately, much was known about the snow skink’s life history and how changes in thermal conditions affect the fitness of females and males. This allowed us to construct mechanistic models for the evolution of sex determination, and to parameterize these models with data of a well-studied species.
Theoretically, whether TSD is selectively favoured over GSD depends on the balance of two opposing forces. On the one hand, sex differences in the fitness consequences of timing of birth could favour integration of temperature-dependent developmental processes and gonad differentiation to ensure a match between offspring sex and birth date. On the other hand, sufficiently strong temperature fluctuations between years select against TSD, since years with extreme temperatures would cause very biased sex ratios in a TSD population, giving the stable sex ratios guaranteed by GSD the upper hand.
We constructed detailed models of the sex-specific life histories of both highland and lowland snow skink populations, and in combination with field data from many years we used these to estimate how birth date affects fitness of males and females. It turned out that male fitness was hardly affected by birth date, for both populations, but for females there was a clear advantage of being born early in the lowland population, while no or hardly such advantage was present in the highland population. This implies a selective advantage for TSD in the lowland population, but not in the highland population. However, in both populations, temperatures fluctuated considerably between years, albeit more strongly so in the highland. This tends to select against TSD, so the question remained what the net selective effect on TSD vs. GSD was. To answer this question required detailed evolutionary models, integrating both aspects.
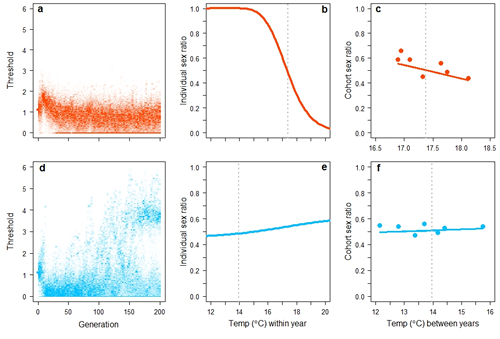
Some simulation results are shown in Figure 1. The model generated two primary results, both in close accordance with empirical data. First, in simulations parameterized with data from the lowland population, sex determination evolved from pure GSD towards a system with a strong temperature effect (Figure 1b). This generated a significant negative correlation between the cohort sex ratio and average temperature during gestation that closely resembled data from the natural population (Figure 1c). Second, in simulations parameterized with data from the highland population, sex chromosomes of the initial GSD system were either retained or, if lost, were replaced by a novel genetic element of major effect via disruptive selection (Figure 1d). Consequently, the model could generate evolutionary shifts from one sex chromosome system to another – including transitions between male and female heterogamety - but it always produced a sex-determining system that generated average sex ratios that did not deviate substantially from equality, again in close accordance with the natural population (Figure 1e,f). These results were robust with respect to starting settings, male versus female heterogamety, and linkage between genetic elements
Climate driven population divergence in sex-determining systems emphasizes a creative role of phenotypic plasticity in evolution. First, the effect of climate on lizard life history is largely a passive result of how thermal opportunity constrains activity patterns rather than an evolved adaptation. However, such non-adaptive plasticity apparently can be an important source of variation in selection on seasonal sex ratio adjustment and, hence, sex-determining mechanisms across species’ distributions. Second, the observation that stressfully high or low temperatures have a causal effect on sex determination also in vertebrates with GSD suggests that temperature-induced developmental plasticity can simultaneously expose variation in sex determination and cause novel selection on this variation, thereby greatly facilitating evolutionary divergence in sex determination. If so, transitions in sex-determining systems may only require minor secondary modifications, loss, or gain of regulatory elements involved in gonad differentiation, suggesting substantial scope for interchangeability between genetic and environmental determinants of sex.
