Sex ratio selection and multi-factorial sex determination in the housefly
Sex determination is a fundamental developmental process in animals and plants and one might therefore expect the underlying mechanisms to be conserved. Yet the opposite is true: sex determining mechanisms vary considerably between closely related taxonomic groups and evolutionary transitions from one system to another seem to occur frequently. It is still far from clear why sex determining mechanisms are so evolutionarily unstable and what forces are responsible for their rapid turnover rate. One reason for the lack of this knowledge is presumably that sex determining mechanisms are usually fixed in individual species, but the housefly (Musca domestica) is one of few exceptions. In this species, several different SD mechanisms have been found to co-exist in field populations (Table 1). In the so-called standard XY strains, a male-determining factor (M) is located on the Y chromosome and males are XY and females XX. The M factor blocks the action of an autosomal F which is necessary for female development. In addition to the standard XY system, field populations have been discovered in which an M factor is located on one or several of the five autosomes, or even on an X chromosome. These autosomal (more precisely, non-Y) M factors seem to have appeared relatively recently and may be spreading, replacing the standard XY system in many locations. In most populations with autosomal M factors, an additional epistatic factor FD (FDominant ) occurs, dictating female development, even in the presence of several M factors.
|
Autosomes
IV |
I - V |
Sex chromosomes
XX |
XY |
|---|---|---|---|
| F/F | +/+ | ♀ | ♂ |
| F/F | •/M | ♂ | ♂ |
| F/FD | •/• | ♀ | ♀ |
Table 1: Relation between genotype and sexual phenotype in the housefly. The female determining factors (F/FD ) are located on chromosome IV; the male determining factors (M) can be located on any chromosome. A "+" symbol indicates the wild type state (no M) and a dot the presence or absence of M.
Whatever the causes for the variability and distribution of SD mechanisms in the natural populations the housefly, this organism is potentially very suitable for conducting experimental studies on the evolution of sex determination, and we are currently embarking on such studies. However, in addition to carrying out experiments, it is useful to obtain more theoretical insight into the behaviour of the housefly system.
We investigated the effect of sex ratio selection on the dynamics of the three-locus sex determining system. The reason is that the selection for or against biased sex ratios is thought to be, at least theoretically, an important contributing factor in evolutionary transitions between SD systems. We focused on the most basic mechanism where sons and daughters differ in how much they "cost" to produce by the parents. Selection will then act on genes affecting the sex ratio to favour overproduction of the "cheaper" sex.
We modelled the dynamics of a sex determination system consisting of three gene loci on three different chromosomes, each locus having two possible alleles. The first locus corresponds to the standard XY sex determination system, having an X "allele" and a Y (male-determining) “allele”. The second locus has a male-determining M allele and a neutral "+" allele. The third locus has an epistatic female-determining FD allele and a standard F allele. We made a system of recurrence equations to analyze frequencies of different genotypes in males and females under sex ratio selection. Where possible, we used analytical methods to analyze the equations, but in most cases we had to use numerical iterations. To investigate dependence on initial conditions, for each parameter combination 200 random initial genotype frequencies were sampled.
If there are no cost differences between sons and daughters, it can be shown analytically that all equilibria of the system have an even sex ratio. Equilibrium is reached usually within 10 generations and frequencies of different sex determining factors depend on their initial frequencies, but polymorphism on all of them is maintained.
When daughters are more costly and male-biased sex ratios are selectively favoured, surprisingly, the equilibrium primary sex ratio is always even. The FD factor is always removed from the population, since females with FD often produce female-biased sex ratios and have a selective disadvantage. The system reduces to a population with a mixture of X, Y and M (Figure 1), but without FD in the populations only males heterozygous for M or Y stay in the population, which leads to a 50:50 sex ratio under Mendelian segregation.
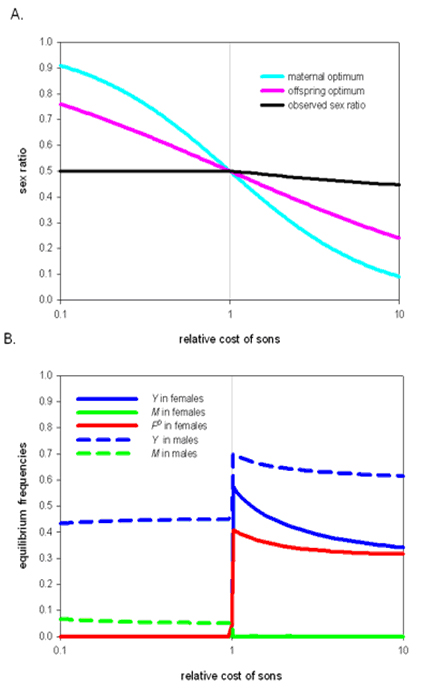
When males are more costly, female-biased sex ratios are expected to be selectively favoured and this is indeed what we found. However, the magnitude of the sex ratio bias is relatively small compared to the expected values, even in situations where sons are much more expensive to produce than daughters (Figure 1). Only a single male-determining factor can remain in the population (either M or Y), the one which was initially present at a higher frequency. FD never reaches a frequency of 0.5 among females; hence a fully female heterogametic system does not evolve.
We have shown that under many circumstances polymorphism on multiple sex determining loci is stable, therefore a wide variety of sex determining mechanisms in natural populations of the housefly may represent an equilibrium state and not, as believed previously, a transient state between systems with only one sex determining factor. Our results show also that stable sex ratio biases, optimal under cost differences between sons and daughters, are not feasible in the housefly sex determining system. These results highlight the potential importance of the constraints imposed by genetic mechanisms on the precision and magnitude of adaptation.
Little is known about the sex ratios and the importance of sex ratio selection in the natural populations of the housefly. It is possible that male biased sex ratios are favoured since adult females are larger than adult male houseflies and presumably need more food. On the other hand, female biased sex ratios are expected under inbreeding which could happen in local populations when they are decreased under unfavourable climatic conditions. However, in view of our results, it seems unlikely that sex ratio selection alone can explain the observed frequencies of M and FD in natural housefly populations. In most populations with non-standard sex determining systems M and FD co-occur, both at high frequencies which is consistent with our model predictions only if sons are more costly than daughters and M has a high initial frequency. Since the XY system seems to be ancestral, high initial frequencies of autosomal M requires additional mechanisms. They could include linkage with fitness enhancing genes or meiotic drive factors, but their incorporation in the model is left for the future.
Although at the moment it is hard to judge whether sex ratio selection has been an important cause of the remarkable variation in housefly sex determining mechanisms, the housefly can still serve as a useful model organism for experiments on the evolution of sex determination. Our model and future theoretical work will be important for designing and understanding the experiments.
