Sexual selection and sympatric speciation
In the last couple of years there has been a revival of interest in the process of speciation. In particular, it has become clear that speciation might to a much larger extent be governed by adaptive (and, hence, predictable) processes than envisaged by classical speciation theories, where speciation is usually assumed to be initiated by external factors, such a geographical segregation. Two recent theoretical developments, from different lines of ../../index, have alleviated two longstanding difficulties in the theory of sympatric speciation. First, sympatric speciation requires, almost by definition, the evolution of a specific mating structure enabling reproductive isolation. Classical models had problems to explain the evolution of assortative mating under general and plausible conditions. A suite of new models demonstrates that these problems can be overcome if sexual selection is the driving force behind the evolution of reproductive isolation. Second, reproductive isolation is not sufficient to ensure the sympatric coexistence of daughter species. These incipient species can only survive if reproductive isolation is associated with ecological differentiation. Only recently, evolutionary branching theory has provided a plausible mechanism for the evolution of ecological differentiation in the presence of disruptive selection. Based on these new insights, a new generation of "ecological" and "sexual selection" models of sympatric speciation has been developed. The two ../../index lines focus on two different aspects (ecological differentiation and reproductive isolation), which are both crucial for a full understanding of speciation. Yet, both lines have largely been developed in isolation.
To provide a conceptual bridge between the two lines, we developed and analyzed a combined model, which, for the first time, integrates the ecological and sexual selection aspects of sympatric speciation (Van Doorn & Weissing, 2001; see Fig. 1). Using a combined analytical and individual-based simulation approach, we were able to show that sympatric speciation is feasible, and that it indeed requires the simultaneous development of ecological differentiation ("ecological branching") and assortative mating ("mating type branching"). Both types of evolutionary branching can be understood as the outcome of a competition process in which individuals compete for a spectrum of either ecological resources or mating opportunities.
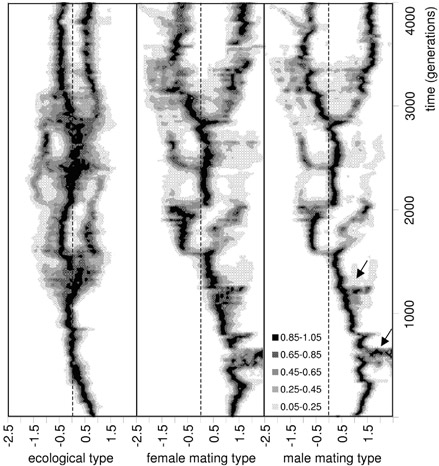
Our analysis highlights that an integrated “eco-evolutionary” view is required to arrive at a really convincing theory of speciation. Keeping this basic insight in mind, we then specifically focused on the role that sexual selection plays in this context. In fact, several questions had not yet been answered satisfactorily by our speciation model. Like all other sexual selection models of sympatric speciation, our model required a high variation in female preferences to obtain divergence of male mating types. In our model, this variation was the result of mutation-selection balance, i.e. of a combination of high mutation rates and weak selection. Other models assumed a dramatic change in the environment, exposing previously hidden preferences to selection. These and similar assumptions are not very satisfactory in that they, again, require external or non-adaptive factors to get the process of sympatric speciation started.
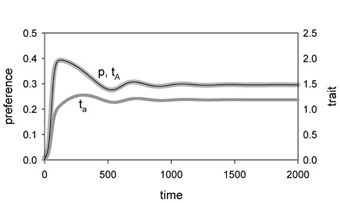
To explore the possibility of a truly “adaptive” road towards sympatric speciation, we investigated a model where the variation in female preferences could be caused by diversifying selection, rather than by mutation or by external events (Van Doorn, Dieckmann & Weissing, 2004). By means of a general argument, verified by individual-based computer simulations, we could show that selection on female preferences has to be frequency dependent in order to get divergence of male and female mating types. If the fitness of females only depends on their own preference, and not on the preferences of other females, mating type branching cannot occur, and the population will always end up in a monomorphic state with one type of preference and the corresponding male mating type (see Fig. 2).
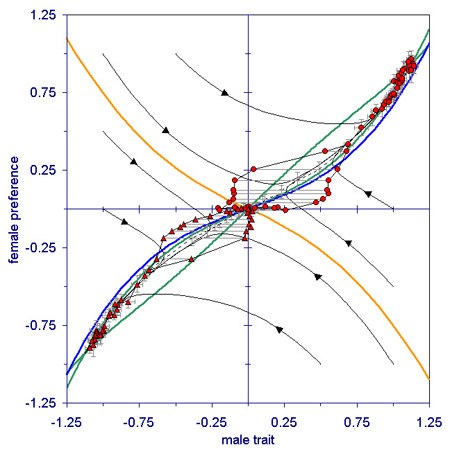
Hence, selection on female preferences has to be disruptive and frequency dependent to initiate reproductive isolation and speciation. However, disruptive frequency dependent selection on female preferences is not sufficient to achieve the simultaneous divergence of female preferences and male mating types. On the contrary, we could show that for a broad class of models branching of female preferences and branching of male mating types exclude each other: whenever selection on female preferences is disruptive, selection on male mating types is stabilizing, and vice versa. From this, one may conclude that truly adaptive speciation by sexual selection is much less easy to achieve than many current models seem to suggest.
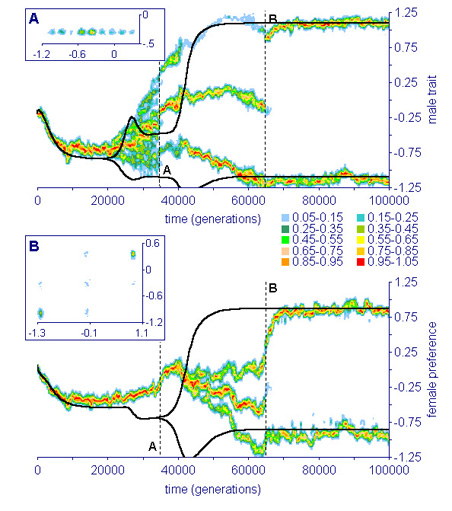
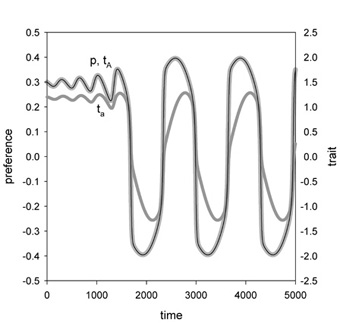
In principle, however, adaptive speciation by sexual selection is possible, provided that several disruptive forces are working in concert. We could demonstrate this by building the first truly adaptive model of sympatric speciation (Van Doorn, Dieckmann & Weissing, 2004; see Fig. 3). To this end, we added male-male competition as an additional disruptive factor into our model. The model structure was motivated by empirical findings on cichlids, where female choice and male-male competition are closely interrelated. Our model is, therefore, perhaps not too far removed from real-world situations. But it clearly indicates that sympatric speciation is not easy to achieve, and that much more ../../index is required to fully understand the emergence of reproductive isolation in sympatry.
Sympatric speciation theory has, up till now, mainly considered Fisherian sexual selection as a potential source of divergence in sexually selected traits. In principle, however, there is a possibility that not only “arbitrary” preferences diversify in the course of evolution, but also preferences for indicators of male quality. Here one might think of systems with multiple ornaments, where different preferences might give different weights to the various ornaments. To investigate this possibility, new theory had to be developed (Van Doorn & Weissing, 2004), since the existing models for the evolution of preferences for multiple ornaments are not very satisfactory. In particular, present models predict that only one of the ornaments can be an indicator of quality, while the other ornaments are “arbitrary”, i.e., maintained by Fisherian runaway selection. In contrast to earlier models, we considered the possibility that different ornaments provide information about different aspects of male quality (e.g., resistance to parasites vs. foraging ability). In other words, the ornaments in our model act as signals for distinct quality components. When the ornaments provide overlapping information about these quality components, we retrieve the results of earlier models. However, when the ornaments provide independent information, preferences for multiple ornaments may evolve by a “good genes” process, even when exhibiting multiple preferences is costly (see Fig. 4). This is good news for behavioural ecologists, who in view of empirical counter-evidence tend to distrust the earlier theoretical results. We will take our new insights as a starting point for a re-evaluation of the role of sexual selection on quality indicators in the process of speciation.
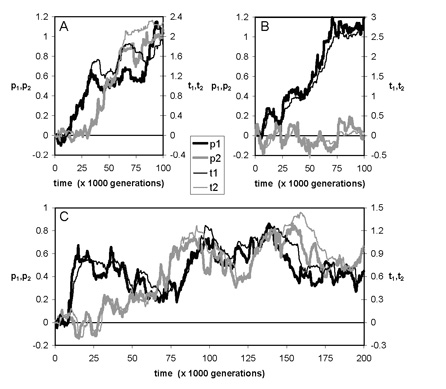
In the course of our work on multiple ornaments we made another observation that is potentially of great importance for the whole theory of sexual selection. Several general results of the theory rely on the assumption that female preference and male ornament expression eventually converge to a stable equilibrium. Examples of such results are the conclusion that no stable preference can evolve for ornaments that are purely epistatic indicators, or the conclusion that females should disregard all ornaments except the one that provides the most reliable information about genetic quality. We showed that the assumption of equilibrium dynamics is problematic and we characterized conditions under which female preference and male condition-dependent signaling continue to evolve without ever attaining stable equilibrium levels. Such limit cycles had been described before in the context of Fisherian runaway selection, but now we demonstrate that continual evolution can also be driven by the joint action of a “good genes” process and a sexual conflict over the information content of signals used in mate choice. Although the existence of this conflict has long been acknowledged, its consequences had never been investigated. Our model illustrates that non-equilibrium dynamics may have major implications, since many of the standard results of sexual selection theory (including those mentioned above) do no longer apply away from equilibrium. The model also provides a mechanism for the apparent frequent loss of sexually selected traits, and it offers an alternative explanation the evolution of preferences for multiple ornaments. Moreover, the model illustrates the importance of genetic constraints on the quantitative and qualitative outcome of sexual selection (see Fig. 5). It is intriguing to see how strongly the new results parallel those in a very different ../../index line, where we showed earlier (in the context of resource competition) that many seemingly well-established results in ecology and evolution only hold if the system does indeed settle on an equilibrium (Huisman & Weissing 1999, 2001ab, 2002, Scheffer et al. 2003).
In addition to female choice, we also addressed male-male competition, the second major aspect of sexual selection and an important ingredient in some of our speciation models (e.g. Van Doorn & Weissing, 2004). In two articles (Van Doorn, Hengeveld & Weissing, 2003ab), we addressed the question why dominance hierarchies do exist, why they are stable, and why one often observes “winner and loser effects”. In many species, difference in dominance rank is used as a cue to resolve conflicts between two animals without escalated fights. At the group level, adherence to a dominance convention efficiently reduces the costs associated with conflicts, but from an individual’s point of view, it is difficult to explain why a low ranking individual should accept its subordinate status. This is especially true if, as suggested by several authors, dominance not necessarily reflects differences in fighting ability but rather results from arbitrary historical asymmetries. According to this idea, rank differentiation emerges from winner and loser effects, in which winners of previous conflicts are more likely to win the current conflict, whereas the losers of previous conflicts are less likely to do so.
By means of game theoretical models, we investigated whether and under what conditions dominance, based on winner and loser effects, can indeed be evolutionarily stable. Even in highly simplified scenarios we found a multitude of alternative evolutionarily stable strategies (ESS). All these strategies utilize the asymmetries generated by the outcome of previous conflicts as cues for conflict resolution. One class of these strategies is based on winner and loser effects, thus generating evolutionarily stable dominance relations even in the absence of differences in strength or fighting ability. However, in view of the fact that alternative conventions are also evolutionarily stable, the question why winner and loser effects seem to occur so often in natural systems is by far not resolved. Still, our game theoretical results provide an interesting link to the ../../index line of Hemelrijk (see Progress Report 2004: "Understanding social behaviour with the help of complexity science" by C.K. Hemelrijk), who uses winner and loser effects as a starting point for the explanation of the various features of egalitarian and despotic societies.
