Photosystem I
Photosystem I in cyanobacteria
Cyanobacteria are abundant throughout most of the world’s water bodies and contribute significantly to global primary productivity through oxygenic photosynthesis. This reaction is catalysed by two membrane-bound protein complexes, photosystem I and photosystem II, which both contain chlorophyll-binding subunits, functioning as an internal antenna. No additional membrane-bound light harvesting systems have been found under normal growth conditions. Iron deficiency, which is often the limiting factor for cyanobacterial growth in aquatic ecosystems, leads to the induction of additional proteins such as IsiA. Although IsiA has been implicated in chlorophyll-storage, energy absorption and protection against excessive light, its precise molecular function and association to other proteins is unknown. In collaboration with Dr. J. Kruip (Ruhr-Universität Bochum and Prof. E. Pistorius, Germany) we have purified a specific PSI-IsiA supercomplex, which abundantly appears under iron-limitation, from two different types of cyanobacteria. Biochemical characterisation showed that the new complex contained multiple copies of IsiA.
Imaging of the purified PSI-IsiA complex by transmission electron microscopy revealed a circular-shaped flat particle with a diameter of 34.5 nm (Fig. A). A data set of top-view projections was analysed by single particle image analysis, which revealed that the novel complex is composed of a central trimeric PSI complex surrounded by a ring of 18 densities. As IsiA is the only additional protein in our preparation, it must form the ring around PSI. Classification of projections indicated that the main variation in the data set was the amount of tilting. Well preserved three-fold rotational symmetry, which is to be expected from zero-tilted particles, was only present in limited numbers of projections (Fig. B), indicating that about 95% of the particles are slightly tilted (Fig. C,D). The inner PSI trimers show a handedness, which is the same as observed in the standard PSI trimers, which also showed numerous tilted projections. This indicates that the bulky stromal PSI surface, composed of the hydrophilic subunits PsaC, PsaD and PsaE, is attached to the carbon support film. Apparently this 4 nm high stromal ridge and the rather flat ring of peripheral IsiA subunits together cause the slight tilt of the majority of particles.
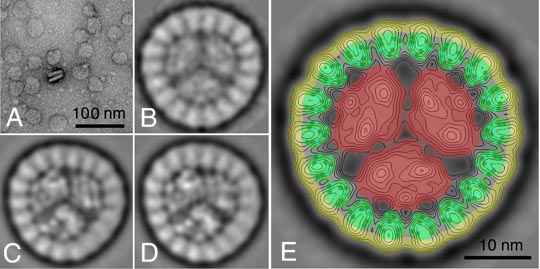
The peripheral IsiA ring could be further interpreted by comparison with the CP43 subunit of the Photosystem II structure, because the IsiA protein is homologous to this subunit. Its projected density is expected to have a very similar surface and has been modelled into the untilted projection (Fig. E). It nicely fits into each of the 18 peripheral densities, if the periphery of the PSI-IsiA complex is corrected for an attached detergent shell. From this fit, it can definitely be concluded that the novel complex is composed of an unusual ring of 18 IsiA proteins. Our data gave the first clear structural characterisation of a photosystem complex from cyanobacteria with a peripheral membrane-bound antenna.
| Laatst gewijzigd: | 03 oktober 2012 11:40 |
