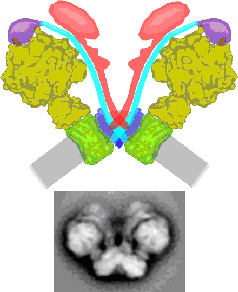
Respiratory chain supercomplexes in mitochondrial membranes
Mitochondria have an intricate heavily folded inner membrane which houses the respiratory chain complexes which are, together with the ATP synthase complex, responsible for energy production stored as ATP. The structure of the individual membrane-bound protein components have been well characterized, especially by X-ray diffraction studies. During the past years increasing evidence that these components are organized into supercomplexes was obtained, particularly by the application of Blue-native polyacrylamide gel electrophoresis. We use single particle electron microscopy for a structural characterization of some of these supercomplexes. Up till now we characterized the supercomplex composed of monomeric Complex I and dimeric Complex III from Arabidopsis (dutch: Zandraket) and more recently the dimeric complex of ATP synthase from Polytomella (a colorless alga related to Chlamydomonas). The ATP synthase is substantially kinked in its membrane-embedded domains and this allows also for the first time to define a functional role for these supercomplexes. The dimeric ATP synthase complex appears to be responsible for the folding of the inner mitochondrial membrane.