ATPase
Two stators visualized in V-type ATPase
In all organisms ATP serves as a major currency of metabolic energy for driving energy dependent processes. F-type and V-type ATPases are small rotary motors that utilize electrochemical ion gradients (H+ or Na+) to synthesize ATP, or vice versa. Both types consist of a headpiece, F1 or V1, which is connected via a stalk region to the membrane-bound part, F0 or V0. A central stalk rotates within a ring of 3 α and 3 ß subunits in F1, or of 3 A and 3 B in V1, in discrete steps of 120° . At the F0/V0 end the central stalk is connected to a ring of c subunits in the membrane. These subunits are believed to rotate against other subunits of F0 and V0, allowing ion translocation at the interface. This implies that additional connection(s), or stator(s), must be present between F1/V1 and F0/V0, preventing futile rotation of the α3ß3 and A3B3 headpiece. To study the stalk region of the V-type ATPase in detail, we analyzed a set of 7500 single molecular projections of the thermophilic bacterium Caloramator fervidus ATPase by electron microscopy. This work is a collaboration with the group of Konings and Lolkema of the microbiology department from the GBB.
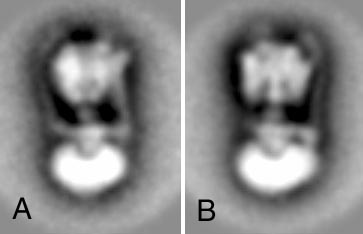
Classification of the projections of detergent-solubilized, negatively stained V1V0, reveals a limited number of preferential orientations, in which V1 is either in a tri-lobed or bi-lobed orientation. The three connections are well separated in the bi-lobed views, as shown in the class-sum of Figure A (left). In the classes with tri-lobed views the left connection is less well resolved, because it is closer to the central stalk and stronger overlapping with V1 (Figure B, right). The two peripheral connections are each attached to an oval-shaped density on top of V1. In some particles, one of the oval densities is absent, and this correlates with the loss of a peripheral connection. From these observations we conclude that the intact V-type ATPase has a central stalk and two stator connections.
Possibly, the two stators are unique for V-type ATPases, because in the stalk region of F-type ATPases only the b subunit is considered to be present in two copies, which are thought to form a dimer, participating in one stator. One could ask why the ATPase motor needs such a complicated stator moiety, which seems to have an overall U-shaped form and which must also avoid unwanted friction with the central rotating stalk. The answer is likely related to a higher mechanical stability of the machinery as a whole.
| Last modified: | 03 October 2012 11.40 a.m. |
