Research Highlights
Prof. dr. Giovanni Maglia
Three top publications 2017-2022
1. Galenkamp N, Biesemans A & Maglia G (2020) Directional conformer exchange in dihydrofolate reductase revealed by single-molecule nanopore recordings. Nature Chemistry 12(5):481-488; DOI: https://doi.org/10.1038/s41557-020-0437-0
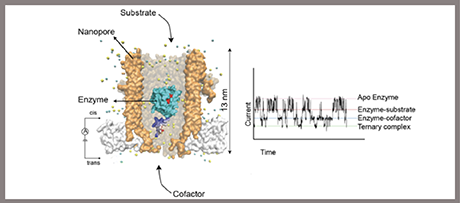
This work showed a remarkable example of single-molecule enzymology using nanopores. It was found that proteins can be electrophoretically trapped inside a nanopore and tiny conformational changes measured by ionic currents. Single-molecule investigations revealed that the enzyme dihydrofolate reductase folds in more than one structure. Like in a two-stroke engine, the free-energy of the chemical step and cofactor binding was used to switch between the two folded structures. It was proposed that this mechanism is used by the enzyme to improve the efficiency of catalysis in high concentrations of oxidized cofactors.
2. Lucas FLR, Versloot RCA, Yakovlieva L, Walvoort TCM & Maglia M (2021) Protein identification by nanopore peptide profiling. Nature Communications 12: 5795; DOI: https://doi.org/10.1038/s41467-021-26046-9
In this work, Giovanni Maglia’s group showed that biological nanopores can be used to study a variety of peptides. In turn, proteins digested into peptides by a protease could be recognized by comparing the resulting ionic current spectra. This approach might allow using biological nanopores in low-cost devices for the analysis and sequencing of proteins at the single-molecule level.
3. Zhang S, Huang G, Versloot RCA, Bruininks BMH, de Souza PCT, Marrinks SJ & Maglia G (2021) Bottom-up fabrication of a proteasome–nanopore that unravels and processes single proteins. Nature Chemistry 13(12): 1192-1199; DOI: https://doi.org/10.1038/s41557-021-00824-w
In this work a biological nanopore was designed from the bottom up from three different proteins. A Bacterial transmembrane pore was fused a Eukaryotic regulatory protein. The latter was then fused to an Archaeal proteasome. The resulting supramolecular complex formed a biological nanopore that allowed the capture, unfolding and processing of single proteins across biological nanopores. This example will allow the analysis and potentially sequencing of proteins at the single-molecule level.
| Last modified: | 22 September 2023 12.24 p.m. |
