Research Highlight
Dr. Julia Kamenz
Every day billions of cells divide in our bodies. Failures during cell division can lead to the loss or gain of genomic information and result in cell death or disease development, such as cancer. The cell division cycle is a complex and highly dynamic signaling network that – in healthy cells – ensures faithful duplication and distribution of the genomic DNA to the two new daughter cells. Sophisticated surveillance mechanisms – cell cycle checkpoints – detect errors during processes such as DNA replication and chromosome segregation and delay cell cycle progression until these errors have been rectified. Cell cycle checkpoints are often linked to irreversible, switch-like transitions into the next cell cycle phase. Unsurprisingly, these highly safeguarded ‘points-of-no-return’ are central to the control of cell proliferation and as such attractive pharmacological targets for the development of new cancer therapeutics.
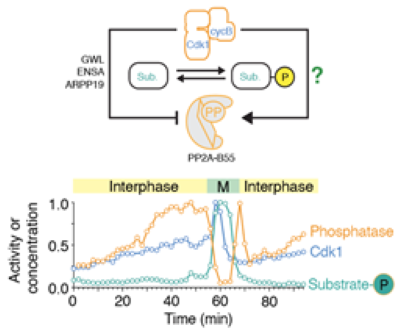
We are using a combination of biochemistry, quantitative cell biology and computational modeling to unravel the complex molecular mechanisms underlying cell division and ensuring error-free cell proliferation. Among other approaches, we are using cycling Xenopus egg extracts that – amazingly – retain the capacity to undergo many of the biochemical processes associated with cell cycle oscillations. Our recent worked highlighted the complex regulation of the phosphatase PP2A-B55 in this process, which is regulated both by incoherent feedforward and double-negative feedback.
Reference
Kamenz J, Gelens L & Ferrell JE Jr. (2021) Bistable, biphasic regulation of PP2A-B55 accounts for the dynamics of mitotic substrate phosphorylation. Current Biology 31: 794-808; DOI: https://doi.org/10.1016/j.cub.2020.11.058
| Last modified: | 24 October 2023 2.29 p.m. |
