Sucking up electrons with acid
Aromatic compounds are very common in nature and millions of tonnes are produced in chemical factories every year. In this case, aromatic doesn’t mean fragrant, but refers to compounds with a ring structure made from carbon atoms. These structures are very stable and flat. But there are also non-aromatic ring structures, which are not flat. Associate Professor of Organic Chemistry Syuzanna Harutyunyan has received a EUR two million ERC Consolidator Grant to develop a method for making 3D structures from 2D rings.

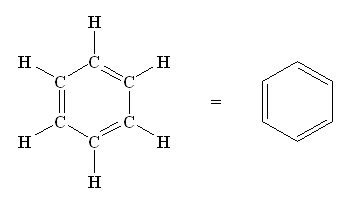
The classic example of an aromatic compound is benzene. This hexagonal structure is made up of six carbon atoms, to which hydrogen is attached. The ring owes its stability to the free electrons that can move along the inside – the signature of an aromatic compound. Chemical reactions in which the aromatic rings remain intact are well known, but this stability makes reactions in which the rings change much more difficult. ‘But three-dimensional molecules with several connected ring systems that are not aromatic are important for making natural products and drugs’, explains Harutyunyan.
‘Organic chemists have to build them step by step from single carbon atoms. This is a shame. It would be much easier to use aromatic compounds as building blocks, but you can’t just change them into non-aromatic rings.’ Both aromatic and non-aromatic rings are very stable, but getting from one to the other requires first destabilizing and then rebuilding the structure. This process is called dearomatization and it can be an efficient way to convert simple, flat aromatic compounds into the complex structures found in drugs.
‘In my project, I will do just that’, says Harutyunyan with confidence. She has done preliminary experiments which show that her idea on how to do this works. To destabilize aromatic rings, Harutyunyan uses what is known as a Lewis Acid. This is any chemical species that attracts free electron pairs. By ‘pulling’ electrons away, the Lewis Acid can make molecules more reactive. This was described in 1923 by American physical chemist Gilbert N. Lewis, hence the name.
‘But it was generally thought that they were not strong enough to destabilize aromatic rings’, explains Harutyunyan. However, she recently used a Lewis Acid to perform another ‘impossible’ reaction and had a hunch they might do the trick with aromatic rings as well. ‘I found a paper from 1985 that described a structure made up of a Lewis Acid and an aromatic ring. That convinced me the acid could react with these rings.’
The Lewis Acid sucks the stabilizing free electrons from the ring. This makes it easier to reduce the aromatic compound to a non-aromatic ring. ‘A PhD student of mine has done a few experiments and it certainly works. But the trick is to make it work exactly the way I want.’ So over the next five years, three PhD students and some postdocs will study ways to make specific structures using aromatics as the starting materials.
‘Ultimately, this might also lead to a better use of biomass’, says Harutyunyan. Lignin, a tough fibre which gives plants their strength, is a polymer made up of different aromatics. The processes developed in the project could be used to break it down into small aromatics, which could be used to make many different chemicals such as medical drugs.
‘But it was generally thought that they were not strong enough to destabilize aromatic rings’, explains Harutyunyan. However, she recently used a Lewis Acid to perform another ‘impossible’ reaction and had a hunch they might do the trick with aromatic rings as well. ‘I found a paper from 1985 that described a structure made up of a Lewis Acid and an aromatic ring. That convinced me the acid could react with these rings.’
The Lewis Acid sucks the stabilizing free electrons from the ring. This makes it easier to reduce the aromatic compound to a non-aromatic ring. ‘A PhD student of mine has done a few experiments and it certainly works. But the trick is to make it work exactly the way I want.’ So over the next five years, three PhD students and some postdocs will study ways to make specific structures using aromatics as the starting materials.
‘Ultimately, this might also lead to a better use of biomass’, says Harutyunyan. Lignin, a tough fibre which gives plants their strength, is a polymer made up of different aromatics. The processes developed in the project could be used to break it down into small aromatics, which could be used to make many different chemicals such as medical drugs.
See also: Making robots (and humans) cooperate
| Last modified: | 12 December 2017 10.44 a.m. |
More news
-
13 May 2024
‘The colourful cells of petals never get boring!’
Most people will enjoy colours in nature. However, the interest of evolutionary biologist Casper van der Kooi goes much further: he studies how flowers, birds, butterflies, and beetles get their colours. He also studies how these colours are used...
-
13 May 2024
Trapping molecules
In his laboratory, physicist Steven Hoekstra is building an experimental set-up made of two parts: one that produces barium fluoride molecules, and a second part that traps the molecules and brings them to an almost complete standstill so they can...
-
07 May 2024
Lecture with soon to be Honorary Doctor Gerrit Hiemstra on May 24
In celebration of his honorary doctorate, FSE has invited Hiemstra to give a lecture entitled ‘Science, let's talk about it’ on the morning of 24 May
