Optical Tweezers show the dynamics of histone-DNA interactions
Together with researchers from Seville, Wouter Roos' group used advanced microscopy techniques to study chromatin function. Using optical tweezers, the researchers have shown how the interactions of histones with DNA - called chromatin - are influenced by other proteins. The results have been published in the journal Advanced Science.
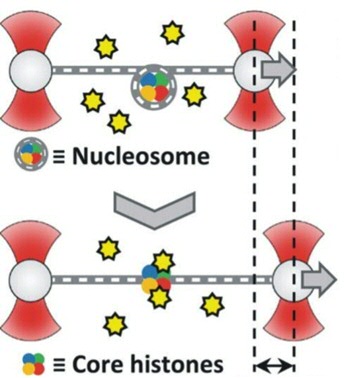
The DNA with attached proteins is called chromatin. This is located in the cell nucleus. Chromatin is made by wrapping DNA around nucleosomes. These are protein complexes consisting of 8 histones. To read the DNA, the DNA must be unwound from the nucleosomes and later rolled up again so that it can be stored compactly in the cell nucleus. In this study, this coiling and uncoiling was investigated and in particular how the interaction with some histones and accessory proteins takes place.
Roos' group used optical tweezers for this. This allows a single DNA molecule to be “suspended” in the liquid using light and you can see live how the DNA interacts with the histones, accessory proteins and how nucleosomes are formed. With this single-molecule technique, the researchers have shown that very different accessory proteins, the chaperones SET/TAF-1β and Nucleofosmin-1, still bind to the histones in the same way and apparently perform their function in a similar way. Furthermore, another protein has been studied, cytochrome c. This protein modulates the way in which the accessory proteins pick the histones from the DNA and thus regulate this process. The results provide a surprisingly detailed look at the activity of the proteins studied and provide new insights into the regulation of the process of DNA reading and coiling around histones.
Publication:
Pedro Buzón, Alejandro Velázquez-Cruz, Laura Corrales-Guerrero, Antonio Díaz-Quintana, Irene Díaz-Moreno, Wouter H. Roos
The histone chaperones SET/TAF-1β and NPM1 exhibit conserved functionality in nucleosome remodelling and histone eviction in a cytochrome c-dependent manner
Advanced Science (2023) 2301859, https://doi.org/10.1002/advs.202301859
More info on the Roos lab:
https://www.rug.nl/research/zernike/molecular-biophysics/roos-group/
| Last modified: | 22 September 2023 11.40 a.m. |
More news
-
21 May 2024
Results of 2024 University elections
The votes have been counted and the results of the University elections are in!
-
13 May 2024
Trapping molecules
In his laboratory, physicist Steven Hoekstra is building an experimental set-up made of two parts: one that produces barium fluoride molecules, and a second part that traps the molecules and brings them to an almost complete standstill so they can...
