New shortcut through nuclear pore complex discovered
Researchers at the Faculty Mathematics and Natural Sciences of the the University of Groningen and of the University Medical Center Groningen have exposed a new shortcut by which membrane proteins are guided to the inner nuclear membrane. Unlike in the method known hitherto, the protein’s ‘address code’ is found to be attached to a long string that wriggles, as it were, through the membrane pore. This discovery is to be published online in the journal Science Express on 9 June at 9 p.m.
The Groningen researchers studied how particular membrane proteins find their way to the right place in a cell. The cell nucleus and the nuclear pore complexes are present in all plant, animal and human cells, and their function is vital to life: if they malfunction, the cell dies. Fundamental research of this kind is an investment in the future and in this particular case will probably have applications in medicine.
Address code
Being in the right place at the right time is very important to a protein: timing and location are crucial if it is to do its job. This is not a new realization, and in the case of many proteins we already know how and when they reach the correct location. Often there is a signal encoded on the surface of the protein: particular transport proteins recognize this ‘address code’ and can then take the protein to the right place in the cell.
Linker
The location that the researchers were interested in is the membrane on the inside of the cell nucleus, the nucleus that also contains the DNA. To get there, proteins need to be guided in via transport channels, the nuclear pores. What the Groningen researchers have unearthed now is an address code completely different from the address codes for transport via the nuclear pores known hitherto. The newly discovered address code is suspended from a long so-called ‘linker’. Cooperation between the first three authors, Anne Meinema, Justyna Laba and Astri Hapsari, was the key to success. The code is specific to certain proteins in the membrane around a cell nucleus.
Shortcut
The long, flexible linker is able to extend and fold up easily and thus to take the address code a long way from the membranes. The linker wriggles its way through the nuclear pore complex so that the address code, to which the transport proteins are attached, can pass through the channel efficiently. Thus a new shortcut through the nuclear pore complex has been discovered.
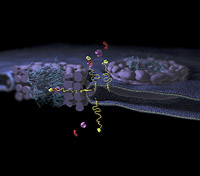
Illustration
Shown here are two nuclear pore complexes (purple and blue) in the membrane around the nucleus and the route that a membrane protein (yellow) takes to reach the inside of the nucleus. It can be seen how how transport proteins (red and purple) recognize the address code at the end of the long linker. The linker wriggles its way through the nuclear pore complex so that the transport proteins can pass through the channel.
More information
• Contact: Dr Liesbeth Veenhoff, tel. 050-363 4187; l.m.veenhoff rug.nl
• Article: Long Unfolded Linkers Facilitate Membrane Protein Import Through the Nuclear Pore Complex in Science, 9 June 2011
• Authors: Anne C. Meinema, Justyna K. Laba, Rizqiya A. Hapsari, Renee Otten, Frans A. A. Mulder, Annemarie Kralt, Geert van den Bogaart, C. Patrick Lusk, Bert Poolman, Liesbeth M. Veenhoff
• Departments involved: Biochemistry and Biophysical Chemistry, University of Groningen; Neuroscience, University Medical Center Groningen; Cell Biology, Yale School of Medicine, USA
| Last modified: | 13 March 2020 01.53 a.m. |
More news
-
13 May 2024
‘The colourful cells of petals never get boring!’
Most people will enjoy colours in nature. However, the interest of evolutionary biologist Casper van der Kooi goes much further: he studies how flowers, birds, butterflies, and beetles get their colours. He also studies how these colours are used...
-
13 May 2024
Trapping molecules
In his laboratory, physicist Steven Hoekstra is building an experimental set-up made of two parts: one that produces barium fluoride molecules, and a second part that traps the molecules and brings them to an almost complete standstill so they can...
-
07 May 2024
Lecture with soon to be Honorary Doctor Gerrit Hiemstra on May 24
In celebration of his honorary doctorate, FSE has invited Hiemstra to give a lecture entitled ‘Science, let's talk about it’ on the morning of 24 May
