Using polymers to squeeze oil from reservoirs
Roughly half the available oil can be recovered from the average oil well. Pumping in water laced with polyacrylamide makes it possible to squeeze more oil from the sandstone, but the high salt concentrations, high temperature and shear force in the reservoir affect this polymer. Chemical Engineer Diego Wever has developed new polymers that may fare better under these harsh conditions.

Wever, who now works at the Shell research facility in Rijswijk, the Netherlands, developed the new polymers during his PhD project at the Department of Product Technology of the University of Groningen. He defended his PhD thesis on 22 November. The Dutch Polymer Institute (DPI) helped fund the project.
You’ve seen it in the cartoons: oil gushes from a hole in the ground, and the flow just needs to be directed into a barrel. Unfortunately, most oil isn’t this eager. You need pumps to pull it out or to inject a gas or liquid to push it out. Normal water doesn’t work here, because pressurized water is too thin to push the oil out of its sandstone hiding place. ‘The water just creates tunnels through the oil’, says Wever.
The solution is to make the water more viscous. This is achieved by adding tons – literally – of polyacrylamide to the water. ‘These molecules are in long, spaghetti-like chains, but they break easily and then lose their ability to increase viscosity.’ Wever therefore set out to see if he could make more efficient polymers.

He wanted to gain some idea of how the new molecules should look, so began with a new approach, which entailed melting different types of commercial polymer and establishing the viscosity of the melt. ‘It turned out that polymers that were more brush-like had higher viscosity than the spaghetti-like ones.’ His next step was to make these polymers under controlled conditions.
This was a big challenge. ‘Acrylamide is very reactive, and it is difficult to control the length of the molecule during polymerization, as well as the formation of side chains.’ He solved the first problem by adapting a polymerization method that had not been applied to acrylamide before, ‘ living polymerization ’. ‘The trick was to find the right conditions, and that meant doing the experiment again and again, with small variations.’
Once he had succeeded in controlling the length of the chains, Wever had to tackle the next step: making a brush rather than spaghetti. ‘We used a backbone that was based on what is known as a polyketone ’, he explains. This molecule was developed in the chemical research lab of the Shell oil company, but development on it was stopped when Shell scaled down its chemical research. ‘However, my supervisor, Professor Ton Broekhuis, had worked on this molecule at Shell before he came to the University of Groningen.’
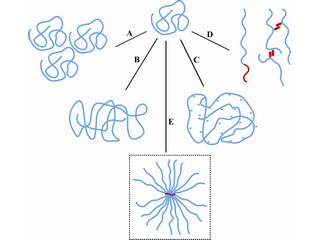
Broekhuis helped Wever devise a method to attach polyacrylamide chains to the polyketone backbone, again in a controlled fashion. ‘So we could now make the brush-like molecule, and control both the number of “hairs” on the brush and their length.’
Finally, Wever needed to test whether the new polymer was, as the melt experiment had suggested, superior to the traditional polyacrylamide spaghetti. And it was. ‘The brush-like molecule is more resistant to high salt concentrations, it doesn’t break as easily as the spaghetti under stress and it is slightly more tolerant to heat.’
Wever also made polymers with building blocks other than acrylamide. ‘When we used isopropylacrylamide, we observed something interesting. At temperatures above 32 degrees Celsius, the isopropylacrylamide becomes hydrophobic, and that affects the characteristics of the polymer.’

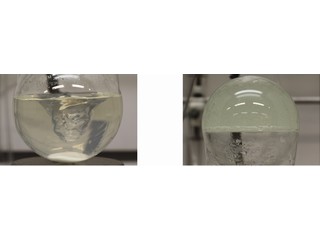
At room temperature, a solution of this mixed polymer has a low viscosity. ‘But at the higher temperatures that are typical of the conditions inside oil reservoirs, the viscosity increases. So we have a “smart polymer”: the polymer solution is easy to inject into the well because it is not very viscous, but once inside the reservoir its viscosity increases.’
Increasing the viscosity is one thing, but the real test was whether the polymer solution could actually push oil from sandstone. Again, the test results were positive. ‘We had a higher yield at lower polymer concentrations. This could save a lot of money and bring up more oil.’ What is more, as Wever can control the shape of his polymers, he could in theory make custom polymers that are optimized to the conditions in specific reservoirs.
Wever’s supervisors Ton Broekhuis and Francesco Picchioni, professors of Chemical Product Technology at the University of Groningen, are full of praise for the work of their PhD student. ‘He has shown that the idea from the melt experiment was correct, has made more resistant polymers and his work has resulted in eight scientific papers, plus a patent on the polymerization technique. And all that in four years!’
Wever’s successor is now working on ways to produce the new polymers at an industrial scale. ‘That’s still quite a challenge’, says Broekhuis. Another challenge is the result of new environmental legislation. The Norwegian government recently passed a law requiring the recovery of the polymers pumped out of an oilfield with the water and oil.
Technologically speaking, this is very difficult and expensive. ‘That’s why it would be a good idea to make biodegradable polymers based, for example, on carbohydrates. We have a lot of knowledge about that in Groningen too.’
Broekhuis has worked at the University of Groningen for ten years now. ‘My role as Professor of Chemical Product Technology has been to train students in product development.’ Traditionally, the chemical engineering programmes have a strong focus on the technical side: building better reactors and other hardware. ‘Our programme is different. We give our students a very strong foundation in Chemistry. We start with the needs of the customer, design those molecules that the customer needs and then develop an industrial production process. The fact that our students are molecule builders sets them apart from other chemical engineers.’
Diego Wever studied Chemical Engineering at the University of Groningen. His PhD project was conducted as part of a research programme of the Dutch Polymer Institute, where researchers work together in the field of polymers. Alongside universities, several oil companies and polymer manufacturers are involved in this programme.
| Last modified: | 02 December 2015 2.40 p.m. |
More news
-
13 May 2024
Trapping molecules
In his laboratory, physicist Steven Hoekstra is building an experimental set-up made of two parts: one that produces barium fluoride molecules, and a second part that traps the molecules and brings them to an almost complete standstill so they can...
-
29 April 2024
Tactile sensors
Every two weeks, UG Makers puts the spotlight on a researcher who has created something tangible, ranging from homemade measuring equipment for academic research to small or larger products that can change our daily lives. That is how UG...
-
16 April 2024
UG signs Barcelona Declaration on Open Research Information
In a significant stride toward advancing responsible research assessment and open science, the University of Groningen has officially signed the Barcelona Declaration on Open Research Information.

