From defect to device
For years, physicists tried to avoid defects when they were growing thin films. But now they love them! University of Groningen Professor of Functional Nanomaterials Beatriz Noheda has just published a paper in the journal Nature, in which she shows how defects can lead to new chemistry.

‘We’re very good at growing thin films at the University of Groningen’, says Noheda. For a long time, that meant growing a layer of perhaps a few dozen atoms that is perfectly flat and uniform. Now Noheda prides herself on being able to create as many breaks in the perfect symmetry of the thin film as she pleases. ‘These films grow as crystals, but crystals can start nucleating at different points on the surface and then grow in different directions. Where two growing crystals meet, they form what is known as a domain wall.’
These walls separate the ‘domains’ of uniform crystals. ‘The walls break the symmetry of the crystal. For years, this was seen as something to be avoided.’ Scientists used all sorts of techniques to grow thin layers that were as uniform as possible. ‘But symmetry breaks are the points where exciting things happen, like in the Big Bang. And matter assumes new properties where the symmetry is broken.’
Tension
Noheda set out to study the broken symmetry in the domain walls. That is no easy task: these walls are just one atom wide. ‘Not even atomic force microscopy has the resolution to study them directly.’ Fortunately, Noheda is good at growing thin films. ‘We can control the number of domain walls by varying the substrate on which the crystals grow and the thickness of the thin layer.’
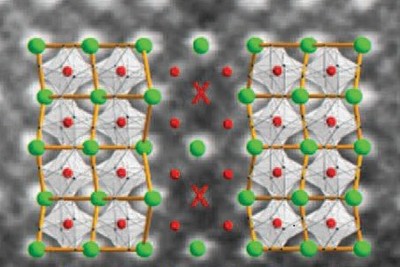
The substrate is a key element: the atomic structure of the substrate layer affects the way in which the crystals grow. Furthermore, the tension inside the thin layers changes with their thickness. ‘These variables help us control the density of domain walls. By comparing stretches with varying wall density, we can correlate properties of the material to the number of domain walls.’
What she noticed in the thin layers described in the Nature paper was that an increase of domain wall density also increased the magnetism of the material. ‘The next question was: how can these domain walls be magnetic, when the domains, the symmetric crystals, are not?’
It took three teams of electron microscopists from Spain, France and Germany to realize that something strange happens at the domains walls. Noheda and her collaborators noticed a change in the crystal structure. This is where things get a little technical. The thin film was grown from a compound called terbium-manganese-oxide. The terbium atoms are bigger than the manganese ones, and they form a sort of zigzag line in the crystal.

Spoon
But something strange happens in the domain wall. Here, the zigs and zags are not neatly aligned, like spoons nested inside a drawer. ‘Instead, they are mirror images of each other’, explains Noheda. So a zig mirrors a zag or in spoon-language: the spoons are arranged with mirroring concave or convex sides. Where two convex sides are opposite each other, the spoons are closer. The same happens in the opposing zigzag lines of terbium atoms. In the domain wall, the distance between two terbium atoms is alternately greater or smaller than in the rest of the crystal.
‘Where two terbium atoms were coming close together in the domain wall, it looked as if there was a void, and the terbium had been squeezed out. But then we found that a terbium atom was in fact replaced by a smaller manganese atom.’ Noheda engaged her colleague Maxim Mostovoy, a professor of theoretical physics at the University of Groningen, and collaborators in Barcelona to model the atomic structure of the domain wall.
Their calculations showed that this manganese was connected to four other manganese atoms in a unique square planar structure. ‘The crystal structure in the domain wall forces the terbium out and creates a new manganese compound. Thus, the stress inside the wall has caused a new chemical reaction to take place.’
That is where it gets really exciting: a new chemical reaction catalyzed by the stress inside a domain wall. ‘This means the domain wall is acting like a chemical reactor at an atomic scale. And we believe what we have found is a general principle. Whenever we have a similar crystal structure, with the zigzag line and a large and small atom, such a replacement can take place.’
Open mind
The discovery may lead to the production of new materials inside domain walls, and that would give the walls new properties. ‘The current material results in magnetism caused by the wall. In the Nature paper, we have described in detail how this magnetism arises, but we can’t do anything useful with it. However, if we can use this knowledge to create walls with a magnetism we can switch at room temperature, it could result in a device of atomic dimensions.’
It makes Noheda think back to her predecessors, who saw domain walls as impurities to be avoided when creating thin films. ‘Their defects may now become new devices. That is a useful lesson for students: always keep an open mind!’
Referentie: Artificial chemical and magnetic structure at the domain walls of an epitaxial oxide. S. Farokhipoor, C. Magén, S. Venkatesan, J. Íñiguez, C.J.M. Daumont, D. Rubi, E. Snoeck, M. Mostovoy, C. de Graaf, A. Müller, M. Döblinger, C. Scheu and B. Noheda Nature, 20 November 2014, DOI 10.1038/nature13918
See also the University of Groningen press release.
| Last modified: | 10 June 2015 2.56 p.m. |
More news
-
16 April 2024
UG signs Barcelona Declaration on Open Research Information
In a significant stride toward advancing responsible research assessment and open science, the University of Groningen has officially signed the Barcelona Declaration on Open Research Information.
-
02 April 2024
Flying on wood dust
Every two weeks, UG Makers puts the spotlight on a researcher who has created something tangible, ranging from homemade measuring equipment for academic research to small or larger products that can change our daily lives. That is how UG...
-
18 March 2024
VentureLab North helps researchers to develop succesful startups
It has happened to many researchers. While working, you suddenly ask yourself: would this not be incredibly useful for people outside of my own research discipline? There are many ways to share the results of your research. For example, think of a...

