Revolution in membrane transport
Cells need to eat, just like you and me. But how do they transport nutrients across their membranes? University of Groningen scientists are the first to discover the mechanism bacteria use to import vitamins, and in the process they discovered that the textbooks were wrong. The results were published in Nature Communications on 30 March.

‘It is quite spectacular’, says Dirk Slotboom, Professor of Biochemistry at the University of Groningen and leader of the research team. ‘This is the culmination of eight years of work.’ He can now show exactly how the bacterial Energy Coupling Factor (ECF) transporter operates. This settles a decades-old debate and points the way to new antimicrobials.
The ECF is a transport system that can shift a host of different substrates through the cell membrane. A more or less universal ECF component joins with a substrate-specific S component. The system is powered by the universal energy carrier ATP. ‘The S component binds the substrate and then translocates through the membrane’, Slotboom explains.
One of the remarkable observations relates to the orientation of the S component. ‘The textbooks provide the standard representation of proteins that are inserted in the membrane’, Slotboom explains. These proteins contain hydrophobic alpha-helices that pass through the membrane, while hydrophilic sections on the outside join these alpha-helices. The orientation was believed to be fixed. ‘But what we saw was that the S component could actually lie horizontally inside the membrane.’
Slotboom discovered that the S component wobbles up and down in the membrane. ‘The acrobatics these membrane proteins can perform are much more spectacular than we thought possible.’ And importantly, they can perform these acrobatics without external energy. ‘It is a pure Brownian motion’, Slotboom explains.
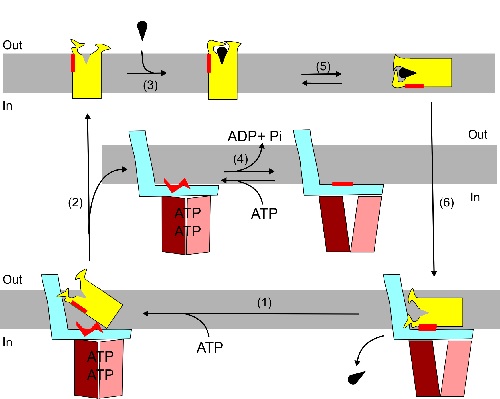
How is this random motion used to transport nutrients? That’s where the ECF component comes in. Slotboom: ‘This protein complex is located on the inner side of the membrane. When the S component with the bound substrate comes into contact with an ECF component, it binds so tightly that this can be considered irreversible.’ The binding causes the S component to release the substrate into the cell. To liberate the now empty S component from the ECF, energy is required, which is where the ATP molecules come into play.
‘The interesting part is that the energy from the ATP is not used to translocate the substrate but to dissociate the S component from the ECF. First the process runs on random Brownian motion, and the ECF acts as a ratchet that ensures the substrate is released inside the cell.’ This settles a debate that has raged for years in the scientific community: is translocation mediated by a ‘power stroke’, like the movement of a piston in a combustion engine, or by a Brownian ratchet? ‘We have shown that in this case it’s the ratchet, and have thus provided fundamental insight into the working of molecular motors in the cell.’
The team pieced together the sequence of events by analysing the protein structures in different phases. ‘Unfortunately, we were scooped three times: with the structure of the S component, the ECF part and the bound state of S and ECF’, says Slotboom. ‘But those studies were done with different substrates, and the groups involved did not reveal the entire translocation process. We analysed all structures of the same folate-specific transporter, so we can now show how it works.’
Elucidating the structure of a protein can be a challenge, especially when it is inserted in a membrane. ‘The classic approach is to grow crystals from the protein and study them with X-ray diffraction, but over the past few years the direct imaging of proteins with high-resolution electron microscopy has given the field an enormous boost.’ Slotboom is working on single-molecule microscopy that uses fluorescent labels for the study of the dynamics of transport. ‘And we’re working on using computer simulations for structural analysis, together with my colleague Siewert-Jan Marrink.’
Another project involves finding a way to block the transport. The ECF system is only present in micro-organisms and not in mammalian cells, so it is an obvious target for the development of antimicrobials. ‘Together with chemist Anna Hirsch, we’re looking for small molecules that will stop the translocation, for example by blocking the step where the ECF ratchet binds to the S component.’ Now that they understand the mechanism, Slotboom and his colleagues will do their best to throw a spanner in the works.
Reference: Lotteke J.Y.M. Swier, Albert Guskov & Dirk J. Slotboom: Structural insight in the toppling mechanism of an energy-coupling factor transporter, Nature Communications, 30 March, DOI 10.1038/NCOMMS11072
| Last modified: | 30 March 2016 11.07 a.m. |
More news
-
16 April 2024
UG signs Barcelona Declaration on Open Research Information
In a significant stride toward advancing responsible research assessment and open science, the University of Groningen has officially signed the Barcelona Declaration on Open Research Information.
-
02 April 2024
Flying on wood dust
Every two weeks, UG Makers puts the spotlight on a researcher who has created something tangible, ranging from homemade measuring equipment for academic research to small or larger products that can change our daily lives. That is how UG...
-
18 March 2024
VentureLab North helps researchers to develop succesful startups
It has happened to many researchers. While working, you suddenly ask yourself: would this not be incredibly useful for people outside of my own research discipline? There are many ways to share the results of your research. For example, think of a...

