Study reveals how bacteria transport vitamins
University of Groningen biochemists led by Professor Dirk Slotboom have revealed how bacteria absorb their vitamin B3. The results of this research may lead not just to new antibiotics but also to the more efficient production of vitamins. The research was published on Wednesday in the journal Nature Structural and Molecular Biology. It is Slotboom’s fifth Nature publication in under two years.
Bacteria also need their daily dose of vitamins. They therefore have special proteins in the cell membrane that help them absorb vitamins from their surroundings into their cells. ‘I am interested in this kind of membrane transport’, says Dirk Slotboom. ‘In particular how cells absorb small molecules.’
Vitamin B3
Slotboom focused his research on the transport of vitamin B3. ‘Little is known about this. There has been some research into it in the past, which gave us some idea of what such a transporter protein might look like.’ Armed with this knowledge, Slotboom scoured microbial genomic databases. ‘There proved to be an extensive family of this kind of protein.’
He decided to focus on the vitamin B3 transporter protein of the bacteria Neisseria mucosa. ‘We could produce sufficient quantities of this protein and it formed good crystals.’ You need these crystals if you want to use x-ray crystallography to elucidate the three-dimensional structure of the protein.
Eureka moment
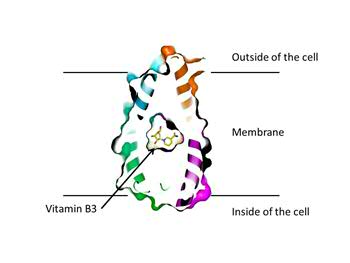
Slotboom: ‘Once you have your results, you can immediately see the structure of the protein. That is a real eureka moment.’ Slotboom could see that the transporter protein known as PnuC weaved through the cell membrane seven times. ‘We also noticed that the first three and the last three segments were completely symmetrical. The fourth segment served to join these two sections.’
The parts sticking out of the inside and outside of the cell membrane were also symmetrical. ‘This could explain the observation that this protein can transport the vitamin B3 just as easily into as out of the cell.’ In nature, the transport is only into the cell, because the vitamin is modified in the cell, preventing it from leaving the cell again.
Meningitis
Slotboom and his colleagues have described exactly how the vitamin B3 binds to the transporter protein. This knowledge should make it possible to produce synthetic molecules that are like vitamin B3 but that halt the transporter. ‘A bacterium such as Haemophilus influenzae, which can cause meningitis, is totally dependent on the PnuC transporter. If you can halt this, you can kill the bacteria.’ It might also be possible to design a toxic molecule that the transporter would carry into the cell.
A third possible application could be in biotechnology. Vitamins currently tend to be produced in chemical synthesis. ‘That is relatively expensive. It would be cheaper to modify bacteria so that they produce and secrete a large quantity of vitamin B3.’
Success
Despite the practical applications, fundamental questions still need answering, and this is where Slotboom would like to concentrate his attention. ‘Now we have a snapshot of the absorption of vitamin B3, we want to reveal the full dynamics of transport through the cell membrane.’
This study is the fifth article by Slotboom, who works at the Groningen Biomolecular Sciences and Biotechnology Institute (GBB) at the University of Groningen, to be published in one of the Nature journals since February 2013. What is the secret of his success? ‘It is difficult to say. We mainly look at systems about which very little is known, and use a whole range of techniques. That seems to appeal to the editors.’
Note to the editor:
- For more information: Professor Dirk Slotboom: tel. 050 363 4187; d.j.slotboom rug.nl
- Referentie: Crystal structure of vitamin B3 transporter PnuC, a full-length SWEET homolog. Michael Jaehme, Albert Guskov & Dirk Jan Slotboom. Nature Structural & Molecular Biology
| Last modified: | 19 March 2020 12.53 p.m. |
More news
-
23 April 2024
Nine MSCA Doctoral Network grants for FSE researchers
Nine researchers of the Faculty of Science and Engineering have received a Horizon Europe Marie Sklodowska Curie Doctoral Network grant.
-
22 April 2024
Charissa Roossien secures JTF subsidy to develop Health Tracker
Dr. Charissa Roossien (ENTEG) has successfully secured a Just Transition Fund (JTF) subsidy of 1.8 million euros to develop a Health Tracker for reliable respiratory and metabolic analysis.
-
16 April 2024
UG signs Barcelona Declaration on Open Research Information
In a significant stride toward advancing responsible research assessment and open science, the University of Groningen has officially signed the Barcelona Declaration on Open Research Information.
